
 25”ꏱ£¬Ļņ20mL0.2mo•lL-1HRČÜŅŗÖŠÖš½„µĪČė0.2mol•L-1NaOHČÜŅŗ£¬µĆµ½ČēĶ¼µÄµĪ¶ØĒśĻߣ®ĻĀĮŠĮ£×ÓÅØ¶ČµÄ¹ŲĻµŹ½“ķĪóµÄŹĒ£Ø””””£©
25”ꏱ£¬Ļņ20mL0.2mo•lL-1HRČÜŅŗÖŠÖš½„µĪČė0.2mol•L-1NaOHČÜŅŗ£¬µĆµ½ČēĶ¼µÄµĪ¶ØĒśĻߣ®ĻĀĮŠĮ£×ÓÅØ¶ČµÄ¹ŲĻµŹ½“ķĪóµÄŹĒ£Ø””””£©| A£® | µć¢ŁČÜŅŗÖŠ£¬c£ØHR£©+2c£ØH+£©=c£ØR-£©+2c£ØOH-£© | |
| B£® | µć¢ŚČÜŅŗÖŠ£¬c£ØNa+£©=c£ØHR£©+c£ØR-£© | |
| C£® | µć¢ŪČÜŅŗÖŠ£¬c£ØNa+£©£¾c£ØR-£©£¾£ØOH-£©£¾c£ØH+£© | |
| D£® | µĪ¶Ø¹ż³ĢÖŠæÉÄÜ»į³öĻÖ£¬c£ØHR£©£¾c£ØR-£©£¾£ØH+£©£¾c£ØNa+£© |
·ÖĪö æŖŹ¼Ź±20mL0.2mo•lL-1HRČÜŅŗÖŠpH=2£¬ŌņHRĪŖČõĖį£¬µ±µĪČė0.2mol•L-1NaOHČÜŅŗĪŖ10mLŹ±£¬ČÜŅŗÖŠČÜÖŹĪŖµČĪļÖŹµÄĮæµÄHRŗĶNaR£¬ČÜŅŗĻŌĖįŠŌ£»µ±Ē”ŗĆÖŠŗĶŹ±ĻūŗÄ0.2mol/LNaOHČÜŅŗ20mL£¬ČÜŅŗĻŌ¼īŠŌ£¬µć¢Ś³ŹÖŠŠŌ£¬ŌņÓŠV£¼20£¬µ«“ĖŹ±c£ØNa+£©=c£ØCH3COO-£©£¾c£ØOH-£©=c£ØH+£©£¬½įŗĻµēŗÉŹŲŗćŗĶĪļĮĻŹŲŗć·ÖĪö£®
½ā“š ½ā£ŗA£®µć¢ŁČÜŅŗÖŠ£¬1NaOHČÜŅŗĪŖ10mLŹ±£¬ČÜŅŗÖŠČÜÖŹĪŖµČĪļÖŹµÄĮæµÄHRŗĶNaR£¬ČÜŅŗÖŠ“ęŌŚc£ØHR£©+c£ØR-£©=2c£ØNa+£©£¬c£ØNa+£©+c£ØH+£©=c£ØR-£©+£ØOH-£©£¬ÓÉ“ĖæɵĆc£ØHR£©+2c£ØH+£©=c£ØR-£©+2c£ØOH-£©£¬¹ŹAÕżČ·£»
B£®µć¢ŚČÜŅŗÖŠ£¬ČÜŅŗĻŌÖŠŠŌc£ØH+£©=£ØOH-£©£¬ČÜŅŗÖŠµēŗÉŹŲŗćĪŖc£ØNa+£©+c£ØH+£©=c£ØR-£©+£ØOH-£©£¬ĖłŅŌc£ØNa+£©=c£ØR-£©£¬¹ŹB“ķĪó£»
C£®µć¢ŪČÜŅŗÖŠ£¬HRÓėNaOHĒ”ŗĆ·“Ӧɜ³ÉNaR£¬ČÜŅŗĻŌ¼īŠŌ£¬Ōņc£ØNa+£©£¾c£ØR-£©£¾£ØOH-£©£¾c£ØH+£©£¬¹ŹCÕżČ·£»
D£®µĪ¶ØæŖŹ¼Ź±£¬ČÜŅŗÖŠHR¹żĮ棬ČÜŅŗĻŌĖįŠŌ£¬ŌņČÜŅŗÖŠæÉÄÜ“ęŌŚc£ØHR£©£¾c£ØR-£©£¾£ØH+£©£¾c£ØNa+£©£¬¹ŹDÕżČ·£®
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²éĖį¼ī»ģŗĻČÜŅŗPHµÄÅŠ¶ĻÓė¼ĘĖć£¬ĢāÄæÄŃ¶Č½Ļ“ó£¬×¢Ņā“Óµē½āÖŹµÄĒæČõŅŌ¼°Ėį¼ī»ģŗĻ·“Ó¦µÄ½Ē¶Č·ÖĪö£¬×¢Ņā·ÖĪöĒśĻߵıä»ÆĢŲµć£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ÓĆĶŗĶĻ”ĻõĖįÖĘȔɣĮæNO | |
| B£® |  ½«»ÆѧÄÜ×Ŗ»ÆĪŖµēÄÜ | |
| C£® |  ·ÖĄė·ŠµćĻą²ī½Ļ“óµÄ»„ČÜŅŗĢå»ģŗĻĪļ | |
| D£® | 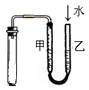 ¼ģŃé×°ÖĆĘųĆÜŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  Öʱø²¢ŹÕ¼ÆCl2 | B£® | 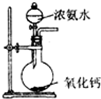 Öʱø°±Ęų | ||
| C£® | 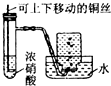 Öʱø²¢ŹÕ¼ÆNO2ĘųĢå | D£® |  ÖʱøŃõĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 CH3COO-+H+£®
CH3COO-+H+£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ħ¶ūŹĒ¹ś¼ŹæĘѧ½ē½ØŅé²ÉÓƵÄŅ»ÖÖĪļĄķĮæ | |
| B£® | ĪŅĆĒ°Ńŗ¬ÓŠ6.02”Į1023øöĮ£×ÓµÄČĪŗĪĮ£×ӵļÆĢå¼ĘĮæĪŖ1Ħ¶ū | |
| C£® | Ħ¶ūŹĒ±ķŹ¾ĪļÖŹµÄĮ£×ÓøöŹżµÄµ„Ī»£¬¼ņ³ĘĦ£¬·ūŗÅĪŖmol | |
| D£® | 1molŃõŗ¬6.02”Į1023øöO2 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com