
ij��ѧ��ȤС���ͬѧ�ǰ��������ʵ�鷽���Ʊ������������壺����ȡ��������ˮ�ڽྻ���ձ��У��þƾ��Ƽ��������ڣ����ձ�����εμӱ��͵�FeCl3��Һ������У���Һ������ĺ��ɫ��
FeCl3��3H2O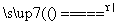 Fe(OH)3(����)��3HCl
Fe(OH)3(����)��3HCl
(1)�жϽ����Ʊ��Ƿ�ɹ��������ý����____________��
(2)�����Ʊ��������������ʵ��ʱ����Щͬѧû�а�Ҫ����У����û�й۲쵽���壬����Ԥ����������ԭ��
�ټ�ͬѧû��ѡ�ñ����Ȼ�����Һ�����ǽ�ϡ�Ȼ�����Һ�����ˮ�У����û�й۲쵽________________����ԭ����____________________________________________��
����ͬѧ��ʵ����û��ʹ������ˮ������������ˮ�������________________________��ԭ����______________________________________________��
�۱�ͬѧ���ˮ�еμӱ����Ȼ�����Һ��ʱ����ȣ������___________________��ԭ����________________________________________��
(3)��ͬѧ��Ҫ���Ʊ���Fe(OH)3���壬����������Fe(OH)3��������μ�����ϡH2SO4��Һ�����������һϵ�б仯��
���ȳ��ֺ��ɫ������ԭ����_______________________________________________��
���������ܽ⣬�˷�Ӧ�����ӷ���ʽ��_____________________________________
______________��
�𰸡�(1)�����ЧӦ
(2)�ٺ��ɫҺ�塡FeCl3��Һ̫ϡ�����ɵ�Fe(OH)3̫��
�����ɺ��ɫ����������ˮ�к��е���ʣ����巢���۳�
�����ɺ��ɫ��������ʱ����Ƚ��巢���۳�
(3)�ٵ����H2SO4ʹFe(OH)3����۳�����������
��Fe(OH)3��3H��===Fe3����3H2O
������(2)������ϡFeCl3��Һ����ˮ�������Fe(OH)3��̫�٣����������ɫҺ�塣������ˮ�к��н϶�ĵ���ʣ�ʹ����۳����۳�ʱ����ȣ����巢���۳���(3)����ϡH2SO4��Fe(OH)3��۳����������ɫ���������H2SO4����Fe(OH)3�����кͷ�Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪C(s)��H2O(g)��CO(g)��H2(g) ��H��akJ��mol��1
2C(s)��O2(g)��2CO(g) ��H����220kJ��mol��1
H��H��O��O��O��H���ļ��ֱܷ�Ϊ436��496��462kJ��mol��1,��aΪ( )
A����332 B����118 C����350 D����130
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Na��S���ʼ��仯��������������б�ŵĻ�ѧ����ʽ��
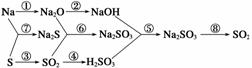
�����йط�Ӧ�Ļ�ѧ����ʽΪ
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķ�����ȷ���� (����)
����� ���ᡡ�� ���Ρ� �� ���������������������
A��Na2CO3 H2SO4����NaOH���� SO2������ CO2
B��NaOH HCl NaCl Na2O NO
C��KOH HNO3 CaCO3 CaO Mn2O7
D��NaOH HCl CaF2 Na2O2 SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� (����)
A��������Ư�۳���������ˮ�ľ�����ɱ�����������ߵ�����ԭ����ͬ
B��ͨ����ѧ�仯����ʵ��235U��238U���ת��
C��ú��������Һ���������������仯���̣��ɱ�Ϊ�����Դ
D��ʯ���ѽ⡢ú����������ˮ��þ�ȹ����ж�������ѧ�仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����е������ʵĵ��뷽��ʽ
(1)H2SO4_________________________________________________________________��
(2)H2CO3_________________________________________________________________��
(3)Ca(OH)2________________________________________________________________��
(4)NH3��H2O_______________________________________________________________��
(5)NaCl___________________________________________________________________��
(6)BaSO4_________________________________________________________________��
(7)NaHSO4________________________________________________________________��
(8)NaHCO3________________________________________________________________��
(9)NaHSO4(����)___________________________________________________________��
(10)CH3COOH_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ca(HCO3)2��Һ��NaOH��Һ��Ӧ
(1)Ca(HCO3)2����________________________________________________________��
(2)Ca(HCO3)2����________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ���� (����)
A����������ˮʱ��Cl2��H2O2H����Cl����ClO��
B���ö��Ե缫��ⱥ���Ȼ�����Һ��2Cl����2H2O===H2����Cl2����2OH��
C����Fe(NO3)3��Һ�м��������HI��Һ��2Fe3����2I��===2Fe2����I2
D��Na2SO3��Һʹ����KMnO4��Һ��ɫ��5SO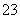 ��6H����2MnO
��6H����2MnO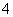 ===5SO
===5SO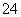 ��2Mn2����3H2O
��2Mn2����3H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com