
���������(Na2S2O3)�����������Լ�������Ļ�ԭ���������ȡ������ֽ⡣��ҵ�Ͽ��÷�Ӧ��2Na2S��Na2CO3��4SO2===3Na2S2O3��CO2 �Ƶá�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ���ش��������⣺

(1)b�з�Ӧ�����ӷ���ʽΪ__________________________________��
c���Լ�Ϊ____________��
(2)��Ӧ��ʼ��c�����л��Dz��������ֱ���塣�˻�������____________��
(3)d�е��Լ�Ϊ______________��
(4)ʵ����Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ��
________________________________________________________________________
___________________________________________________(�����)��
(5)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ��SO2���ܹ�����ԭ����________________________________________________________________________
________________________________________________________________________��
�𰸡�(1)SO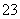 ��2H��===SO2����H2O��HSO
��2H��===SO2����H2O��HSO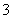 ��H��===SO2����H2O�����ƺ�̼���ƻ����Һ
��H��===SO2����H2O�����ƺ�̼���ƻ����Һ
(2)��
(3)NaOH��Һ
(4)���Ʒ�Ӧ�¶ȡ�������ĵμ��ٶ�(��������Ũ�ȵ�)
(5)��SO2��������Һ�����ԣ�����ֽ�
����������װ��ͼ��֪�������װ�����Ʊ�SO2���м�װ�������Ʊ����������(Na2S2O3)���Ҳ�װ����β������װ��(����SO2)��
(1)b�����Ʊ�SO2��ʵ���ҳ�����������(������������)�����ᷴӦ���ɶ������������ƺ�ˮ�����ӷ���ʽΪSO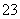 ��2H��===SO2����H2O��HSO
��2H��===SO2����H2O��HSO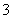 ��H��===SO2����H2O��������ȡ���������(Na2S2O3)�ķ���ʽ2Na2S��Na2CO3��4SO2===3Na2S2O3��CO2����֪c���Լ�Ϊ���ƺ�̼���ƻ����Һ��
��H��===SO2����H2O��������ȡ���������(Na2S2O3)�ķ���ʽ2Na2S��Na2CO3��4SO2===3Na2S2O3��CO2����֪c���Լ�Ϊ���ƺ�̼���ƻ����Һ��
(2)��ΪSO2���������ԣ���Һ�д���S2�������Զ����ܷ���������ԭ��Ӧ���ɵ���S��
(3)d��β������װ��(����SO2)������d��ʢ�ŵ��Լ���NaOH��Һ��
(4)����SO2�������ʣ����Բ�ȡ���Ʒ�Ӧ�¶ȡ�������ĵμ��ٶ�(��������Ũ��)�ķ�����
(5)���������(Na2S2O3)����ǿ�������Σ�������������Ӧ(S2O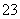 ��2H��===S����SO2����H2O)����SO2����������Һ�����ԣ����������(Na2S2O3)�ͷ�����Ӧ���²�Ʒ�������١�
��2H��===S����SO2����H2O)����SO2����������Һ�����ԣ����������(Na2S2O3)�ͷ�����Ӧ���²�Ʒ�������١�


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������� ��һ���ڸ�������������������FeSO4��Һ��Ӧ�ķ�������֤��Ӧʵ�ʡ�ʵ��ʱ����100 mL���Թ����ȼ�40 mLú�ͣ�ȡ3��������С�Ľ����Ʒ�����Թܺ�������Ƥ����ͨ������©������FeSO4��Һʹú�͵�Һ�������������н����ɼ�(��ͼ)����ϸ�۲죬�ش��������⣺
��һ���ڸ�������������������FeSO4��Һ��Ӧ�ķ�������֤��Ӧʵ�ʡ�ʵ��ʱ����100 mL���Թ����ȼ�40 mLú�ͣ�ȡ3��������С�Ľ����Ʒ�����Թܺ�������Ƥ����ͨ������©������FeSO4��Һʹú�͵�Һ�������������н����ɼ�(��ͼ)����ϸ�۲죬�ش��������⣺
(1)��δ��Լ�ƿ��ȡ�ý����ƣ�ʣ���Na�ܷ�Ż�ԭ�Լ�ƿ��
(2)�й��Ʒ�Ӧ��������__________________________________________��
(3)���Թܵ���Һ�г��ֵ�����______________________________________________��
(4)װ����Һ��ı仯�����Թ���______������©����____��
(5)д����������������Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧʵ����ʵ������Ͳ���ȷ����(����)
A�����з�̪��NaHCO3��Һ��dz��ɫ���Ⱥ��ɫ�������ΪNaHCO3�ֽ�������Na2CO3
B���Ʊ�����ú���У�����Ϊú�Ͳ����Ʒ�����Ӧ���Ʊ�ú���ܶȴ�ú�Ϳ���ʹ�Ƹ���������ˮ����
C���ýྻ�IJ����������Na2O2����֬��������֬��ȼ�գ�˵��CO2��H2O��Na2O2�ķ�Ӧ�Ƿ��ȷ�Ӧ
D���Ƴ��ڱ�¶�ڿ����еIJ�����Na2CO3��ԭ���������������ɵ�Na2O��ˮ�Ͷ�����̼��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�Ǽ���ij��ɫ����A��SO2��CO2�Ļ�������װ��ͼ����Ҫ��ش��������⡣

(1)B�м�����Լ���________��������____________________________________________��
(2)C�м�����Լ���__________��������_______________________________________��
(3)D�м�����Լ���________��������_________________________________________��
(4)ʵ��ʱ��C��Ӧ�۲쵽��������_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ķ�������ijЩ���ӵļ��飬���з�����������ܵ���(����)
A��������Һ�е�SO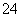 ����Һ
����Һ ����
���� ��ɫ����
��ɫ����
B��������Һ�е�SO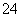 ����Һ
����Һ ����
���� ��ɫ����
��ɫ����
C��������Һ�е�I������Һ ���ɫ��Һ
���ɫ��Һ ���ɫ��Һ
���ɫ��Һ
D��������Һ�е�CO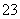 ����Һ
����Һ ��ɫ����
��ɫ���� �����ܽ�
�����ܽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ�����������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2===Na2S2O5��
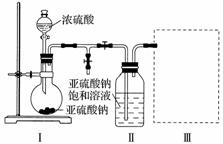
(1)װ�â��в�������Ļ�ѧ����ʽΪ_________________________________________��
(2)Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����___________________________��
(3)װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ__________(�����)��

ʵ��������������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
(4)֤��NaHSO3��Һ��HSO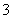 �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
a���ⶨ��Һ��pH
b������Ba(OH)2��Һ
c����������
d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)����Na2S2O5�����ڿ������ѱ�������ʵ�鷽����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������������ȷ���������ϵ����(����)
| ѡ�� | ������ | ������ |
| A | H2�л�ԭ�ԣ�Ũ������ǿ������ | ������Ũ�������H2 |
| B | CuS������ˮ������ | ��Ӧ��H2S��CuSO4===CuS����H2SO4���Է��� |
| C | ŨH2SO4����ˮ�� | ŨH2SO4�����ڸ��ﰱ�� |
| D | SO2�������Ժ�Ư���� | ����ɫʯ����Һ��ͨ��SO2����Һ�ȱ������ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������5�ֻ����е�2����ͬ����������ϣ��γɵ��л�������NaHCO3��Һ��Ӧ����(����)
�١�OH���ڡ�CH3���ۡ�COOH����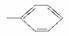 �ݡ�CHO
�ݡ�CHO
A��2�� B��3�� C��4�� D��5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�þ����X�������䷨��Cu�IJⶨ�õ����½����Cu�ľ���Ϊ

�����������ܶѻ�(����ͼ)����֪�þ�����ܶ�Ϊ9.00 g·cm��3�������и�ԭ�ӵ���λ��Ϊ________��Cu��ԭ�Ӱ뾶Ϊ________cm(�����ӵ�����ΪNA��Ҫ����ʽ����)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com