
��10�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��������ԭ�Ӳ�ȡ �ӻ���
��3��д��������AC2�ĵ���ʽ ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ��
��4��ECl3��B��C���⻯���γ���λ��Ϊ���������Ļ�ѧʽΪ ��
��1��C��O��N ��2�֣� ��2�������� ��1�֣� sp3 ��1�֣�
��3�� 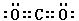 ��2�֣�
N2O ��2�֣� ��4�� [Cr(NH3)4(H2O)2]Cl3 ��2�֣�
��2�֣�
N2O ��2�֣� ��4�� [Cr(NH3)4(H2O)2]Cl3 ��2�֣�
��������B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�˵�������к�����������γ������Ҳ����N��O��F������D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ������C����Ԫ�أ���B�ǵ�Ԫ�أ�D��þԪ�ء�����ΪAC2Ϊ�Ǽ��Է��ӣ����Ըû�������CO2����A��̼Ԫ�ء�
��1���ǽ�����Խǿ����һ������Խ�����ڵ�Ԫ�ص�2p��������ǰ����״̬���ȶ���ǿ�����Ե�Ԫ�صĵ�һ�����ܴ�����Ԫ�صģ���C��O��N��
��2�����������е�ԭ�Ӻ���1�Թ¶Ե��ӣ��������������Σ���ԭ�Ӳ���sp3�ӻ���
��3��CO2�����к���̼��˫���������ʽΪ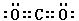 ���ȵ�������ָ��������ԭ�������ֱ���ȵķ��ӣ����Ժ�CO2��Ϊ�ȵ��������N2O��
���ȵ�������ָ��������ԭ�������ֱ���ȵķ��ӣ����Ժ�CO2��Ϊ�ȵ��������N2O��
��4��E��ԭ������Ϊ24����E�Ǹ�Ԫ�ء�������λ����6���Ұ�����ˮ�ĸ���֮����2�U1�����������Ļ�ѧʽΪ[Cr(NH3)4(H2O)2]Cl3��


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com