
 ��
�� ��
�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ij��Һ�м���ϡ���ᣬ����������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�����Һһ����̼������Һ |
| B���ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һһ����������Һ |
| C��Al��Fe��Cu��Ӧ��������������ᷴӦ�����κ�ˮ�����ֽ������������Ϊ���������� |
| D����ij��Һ�еμ���ˮ���ٵμ�KSCN��Һ����Һ��Ѫ��ɫ������Һ�в�һ����Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
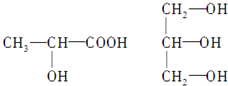
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ӵĵ����Ų�ʽ��1s22s22p63s23p4 |
B��H2O�ĵ���ʽ��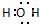 |
C��Nԭ���������ӵĹ����ʾʽ��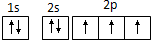 |
D��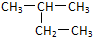 �����ƣ�2-�һ����� �����ƣ�2-�һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��F-�ĵ����Ų�ͼ��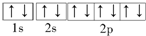 |
B��Na+�Ľṹʾ��ͼ��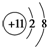 |
| C��Mg2+�ĵ����Ų�ʽ��1s22s22p6 |
| D��Cr�ļ����Ų�ʽ��[Ar]3d44s2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
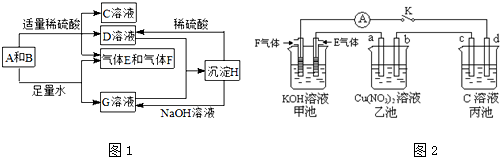
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H3O+ |
| B��SiH4 |
| C��PH3 |
| D��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ���¶��£�ij��Һ��pH��7�������Һ������ |
| B����ˮ�м�������̼���ƹ��彫����ˮ�ĵ��� |
| C��0.02mol?L-1CH3COOH��Һ��0.01mol?L-1NaOH��Һ�������ϣ�����Һ�У�2c��H+��+c��CH3COOH��=2 c��OH-��+c��CH3COO-�� |
| D��Ũ�Ⱦ�Ϊ0.1mol/L��NH4Cl��Һ��NH4HSO4��Һ��ǰ�ߵ�c��NH4+�����ں��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com