
 ¹Ź“š°øĪŖ£ŗ31£®25£»
¹Ź“š°øĪŖ£ŗ31£®25£» ŌĮĻĘųÖŠN2µÄĢå»ż·ÖŹżĪŖ82%£¬¹Ź10m3ŌĮĻĘųÖŠµÄN2µÄĢå»żĪŖ10m3”Į82%=8£®2m3=8200L£¬¹Ź±ź×¼×“æöĻĀµŖĘųµÄĪļÖŹµÄĮæĪŖ
ŌĮĻĘųÖŠN2µÄĢå»ż·ÖŹżĪŖ82%£¬¹Ź10m3ŌĮĻĘųÖŠµÄN2µÄĢå»żĪŖ10m3”Į82%=8£®2m3=8200L£¬¹Ź±ź×¼×“æöĻĀµŖĘųµÄĪļÖŹµÄĮæĪŖ ¹Ź±ź×¼×“æöĻĀ10m3ŌĮĻĘųµÄÖŹĮæĪŖ£ŗ31£®25mol”Į64g/mol+49£®11mol”Į32g/mol+366£®07mol”Į28g/mol=13821£®48”Ö13£®82kg£¬“š£ŗ±ź×¼×“æöĻĀ10m3ŌĮĻĘųµÄÖŹĮæĪŖ13£®82kg£»
¹Ź±ź×¼×“æöĻĀ10m3ŌĮĻĘųµÄÖŹĮæĪŖ£ŗ31£®25mol”Į64g/mol+49£®11mol”Į32g/mol+366£®07mol”Į28g/mol=13821£®48”Ö13£®82kg£¬“š£ŗ±ź×¼×“æöĻĀ10m3ŌĮĻĘųµÄÖŹĮæĪŖ13£®82kg£»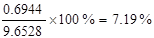
 ÅØĮņĖįÖŠĖ®µÄÖŹĮæĪŖmg-0£®98mg=0£®02mg£¬ĪļÖŹµÄĮæĪŖ
ÅØĮņĖįÖŠĖ®µÄÖŹĮæĪŖmg-0£®98mg=0£®02mg£¬ĪļÖŹµÄĮæĪŖ ¹ŹĪüŹÕČżŃõ»ÆĮņŗóÉś³ÉµÄĮņĖįµÄĪļÖŹµÄĮæĪŖ
¹ŹĪüŹÕČżŃõ»ÆĮņŗóÉś³ÉµÄĮņĖįµÄĪļÖŹµÄĮæĪŖ ·¢ŃĢĮņĖįÖŠĮņĖįµÄĪļÖŹµÄĮæĪŖ0£®01m mol+
·¢ŃĢĮņĖįÖŠĮņĖįµÄĪļÖŹµÄĮæĪŖ0£®01m mol+ 1000m3³öæŚĘųĢåÖŠČżŃõ»ÆĮņµÄĢå»żĪŖ1000m3”Į6£®72%=67£®2m3=67200L£¬SO3µÄĪļÖŹµÄĮæĪŖ
1000m3³öæŚĘųĢåÖŠČżŃõ»ÆĮņµÄĢå»żĪŖ1000m3”Į6£®72%=67£®2m3=67200L£¬SO3µÄĪļÖŹµÄĮæĪŖ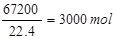 ±»Ė®ĪüŹÕŗóŹ£ÓąµÄČżŃõ»ÆĮņµÄĪļÖŹµÄĮæĪŖ3000mol-
±»Ė®ĪüŹÕŗóŹ£ÓąµÄČżŃõ»ÆĮņµÄĪļÖŹµÄĮæĪŖ3000mol-
 ,½āµĆm=664615g=664£®6kg£¬
,½āµĆm=664615g=664£®6kg£¬

 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
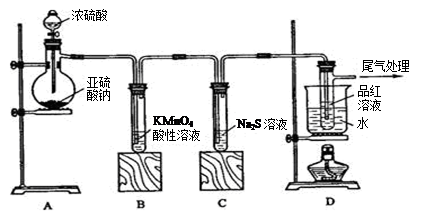
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 Na2SO4£«SO2”ü£«H2O
Na2SO4£«SO2”ü£«H2O
²éæ““š°øŗĶ½āĪö>>
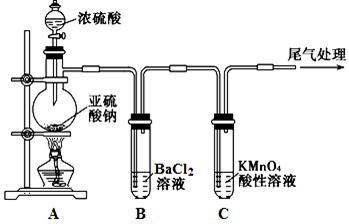
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
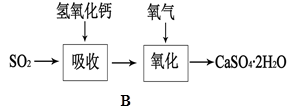
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
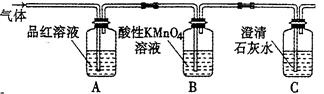
 CO2”ü+2SO2”ü+2H2O£¬ĘäÖŠÅØH2 S04ĖłĘšµÄ×÷ÓĆŹĒ £ØĢī”°Ńõ»Æ¼Į”±»ņ”°»¹Ō¼Į”±£©”£ČōÓŠ0£®2molĢ¼µÄĶźČ«·“Ó¦£¬ŌņĻūŗÄH2S04µÄÖŹĮæŹĒ g£¬±źæöĻĀ²śÉśSO2µÄĢå»żĪŖ______________L”£
CO2”ü+2SO2”ü+2H2O£¬ĘäÖŠÅØH2 S04ĖłĘšµÄ×÷ÓĆŹĒ £ØĢī”°Ńõ»Æ¼Į”±»ņ”°»¹Ō¼Į”±£©”£ČōÓŠ0£®2molĢ¼µÄĶźČ«·“Ó¦£¬ŌņĻūŗÄH2S04µÄÖŹĮæŹĒ g£¬±źæöĻĀ²śÉśSO2µÄĢå»żĪŖ______________L”£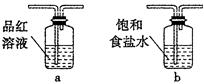
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
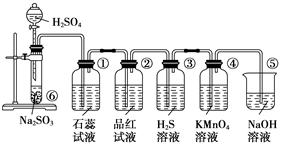 [
[²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®SµÄĘư׊Ō | B£®SµÄ»¹ŌŠŌ |
| C£®SO2µÄĘư׊Ō | D£®SO2µÄ»¹ŌŠŌ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com