
��C1��ѧ����ָ�Է�����ֻ��һ��̼ԭ�ӵ�����Ϊԭ�Ͻ������ʺϳɵĻ�ѧ����C1��ѧ�����ڻ������� ���ص���ԴΣ������������ú����Ȼ���Ȼ�ʯȼ�ϡ����������ȶ��зdz���Ҫ�����塣�ϳ���(CO+H2)�� ��C1��ѧ���еij���ԭ�ϡ�
(1)ú���������ɺϳ������÷�Ӧ�Ļ�ѧ����ʽΪ____________________���ø÷��������ϳ�����һ������ȱ����___________��
(2)�������������ƺϳ�����CH4(g)+1/2O2(g) CO(g)+2H2(g) ��H=-35.6 kJ/mol���÷�Ӧ��_____����Է������Է�������Ӧ��
CO(g)+2H2(g) ��H=-35.6 kJ/mol���÷�Ӧ��_____����Է������Է�������Ӧ��
(3)ͨ���Ҵ���ȡ�ϳ����������õ�Ӧ��ǰ�������Ҵ���ȡ�ϳ�������������·�ߣ�
a��ˮ������������CH3CH2OH(g)+H2O(g)��4H2(g)+2CO(g) ��H=+255.58 kJ/mol
b�����ִ�������CH3CH2OH(g)+1/2O2(g)�� 3H2(g)+2CO(g) ��H=+13.76 kJ/mol
����˵���������____��
A����ԭ�����ĵĽǶ�������a·��������м�ֵ
B�����������ĵĽǶ�������b·�������������
C��a·����������Ҫ���ĺܶ�������������ʵ�����������岻��
D��������������Ӧ�У�ԭ�������ʽϸߵ���b��Ӧ
(4)��ҵ�úϳ����Ʊ������ѵ������������£�

 CH3OH(g) ��H=-90.7 kJ/mol ��
CH3OH(g) ��H=-90.7 kJ/mol �� CH3OCH3(g)+H2O(g) ��H = -23.5 kJ/mol ��
CH3OCH3(g)+H2O(g) ��H = -23.5 kJ/mol �� CO2(g)+H2(g) ��H=-41.2 kJ/mol ��
CO2(g)+H2(g) ��H=-41.2 kJ/mol ��  CH3OCH3(g)+CO2(g)�ġ�H=_____��830�� ʱ��Ӧ�۵�K=1.0�����ڴ���Ӧ���з�Ӧ�۵�K_______���>������<����=����1.0��
CH3OCH3(g)+CO2(g)�ġ�H=_____��830�� ʱ��Ӧ�۵�K=1.0�����ڴ���Ӧ���з�Ӧ�۵�K_______���>������<����=����1.0�� 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
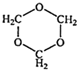
��7�֣���Ȼ��������ָͨ������Ļ�ѧ��Ӧ������һϵ�л�����Ʒ�Ĺ��չ��̣�����C1��ѧΪ��������21���͵���Ҫ������������Ȼ��Ϊԭ�Ͼ����з�Ӧ·�߿ɵù�������PBT��
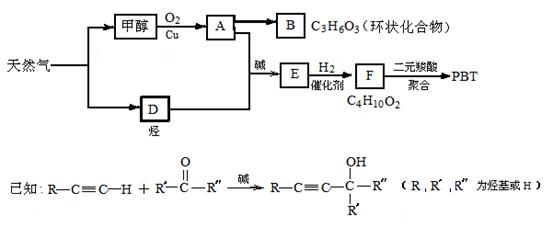
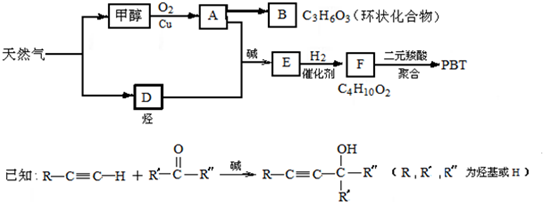
��1��B���ӽṹ��ֻ��һ���⡢һ������һ��̼����B�Ľṹ��ʽ�� ��B��ͬ���칹�����������Ǿ������ƽṹ���� ����д�ṹ��ʽ��
��2��F�Ľṹ��ʽ�� ��PBT���� ���л��߷��ӻ����
��3����A��D����E�ķ�Ӧ����ʽΪ ���䷴Ӧ����Ϊ ��
��4��E��ͬ���칹��G���ܷ���������Ӧ����ʹ��ˮ��ɫ����ˮ���Ҳ����̼ԭ�������ȣ���G��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��1��C1��ѧ��ָ��һ��̼ԭ�ӵĻ������CH4��CO��CO2��CH3OH��HCHO�ȣ������ϳɸ��ֻ�ѧƷ�ļ�������ú����Ȼ���ƺϳ����ٽ�һ���Ʊ����ֻ�����Ʒ�ͽྻȼ�ϣ��ѳ�Ϊ����ѧ��ҵ��չ�ı�Ȼ���ơ����м״���C1��ѧ�Ļ�����
��CO��H2��һ�������������Ҷ�������n(CO)/n(H2)=_____________�������֣���
��������ƽ�������CmHn��ʾ����ϳ����Ϳ���n(CO)/n(H2)=(��m��n��ʾ)��
�ۼ״���һ����������CO��H2���������л���A��A�����Ӿۿ����ɸ߷���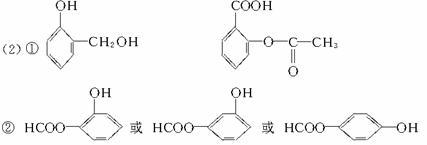 д������A�Ļ�ѧ����ʽ��_________________________________��
д������A�Ļ�ѧ����ʽ��_________________________________��
��2����֪��ȩ��һ���������ܷ������Ϸ�Ӧ��ʾ�����£�
��֪��
��1827�����Ǿͷ����л���A�����ķ���ʽΪC13H18O7����һ����ˮ���ã�ˮ������B��C��
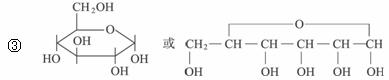
��B�ܷ���������Ӧ��BҲ���ɵ���ˮ��õ���B�ķ���ʽΪC6H12O6��
��C���Ȼ�����Һ�ܷ�����ɫ��Ӧ��1 mol C�������Ʒ�Ӧ�ɲ���1 mol H2��
��C���ʵ������������ʵ��������������ɵ�D��D�ķ���ʽΪC7H6O3����Է�������D��C��14��
��D������ȡ�����������Ǽ�λ������Br2�ڴ��������·���һ��ȡ�������������֣�D����̼��������Һ��Ӧ��
��D����������(CH3CO)2O����Ӧ���ɵó���ҩ��E�����ᣬE����̼�����Ʒ�Ӧ�ų�������̼��
�Իش��������⣺
��д���ṹ��ʽ��C_________________��E_________________��
��д����D��Ϊͬ���칹�壬���б����Һ������ṹ�Ľṹ��ʽ��_________________��ֻ��дһ�֣���
��Bͨ������Ԫ��״�ṹ���ڣ�д��B�Ļ�״�ṹ��ʽ��_________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com