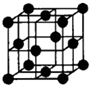
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ���ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�8��Ԫ�أ�D��ԭ��������EС6��D��B���γ����ӻ������侧���ṹ��ͼ����ش�
��1��A��B�γɵĻ������ڹ�̬ʱ�ľ���������
���Ӿ���
���Ӿ���
��A��B�γɵĻ������A��C�γɵĻ������۵�Ҫ
��
��
����ߡ��ͣ�
��2��д��C�ĵ�����ˮ��Ӧ�����ӷ���ʽ
Cl2+H2O=H++Cl-+HClO
Cl2+H2O=H++Cl-+HClO
��
��3����ͼ��ʾ��D��B�γɵ����ӻ�����Ļ�ѧʽΪ
CaF2
CaF2
����������ӻ������Ƿ�Ϊ���壬��ɿ��Ŀ�ѧ������
X�������䷨
X�������䷨
�������ӻ����ᄃ����ܶ�Ϊag?cm
-3��B��D��Ԫ�ص����ԭ�������ֱ�Ϊb��c�����������
cm
3��ֻҪ���г���ʽ����

 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ���ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�8��Ԫ�أ�D��ԭ��������EС6��D��B���γ����ӻ������侧���ṹ��ͼ����ش�
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ���ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�8��Ԫ�أ�D��ԭ��������EС6��D��B���γ����ӻ������侧���ṹ��ͼ����ش�

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
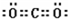
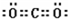
 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�