
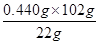 ��2.040 g
��2.040 g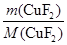 ��
��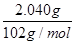 ��0.020 mol
��0.020 mol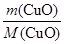 ��
��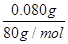 ��0.001 mol��
��0.001 mol��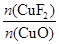 ��
��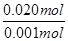 ��20��1
��20��1


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ʣ�࣬��Һ��dz��ɫ��Cl��Ũ�Ȼ������� |
| B������Һ�е�����ɫKSCN��Һ���Ի�ɫ |
| C��Fe2����Fe3�������ʵ���֮��Ϊ6��1 |
| D�����������뻹ԭ��������ʵ���֮��Ϊ2��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
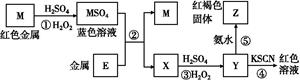
| A��E3+�������Ա�M2+���������� |
| B���ڷ�Ӧ��������ȱ��������ԡ��ֱ����������� |
C����Ӧ�ܵ����ӷ���ʽ�ɱ�ʾΪ:3SCN-+E3+ E(SCN)3�� E(SCN)3�� |
| D���ڢ۷�Ӧ��������ϡ������ܿ������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
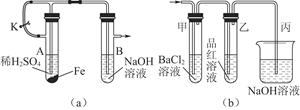
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
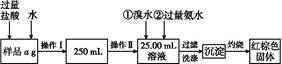
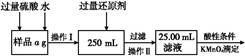
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��3�U14 | B��1�U7 | C��2�U7 | D��3�U2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
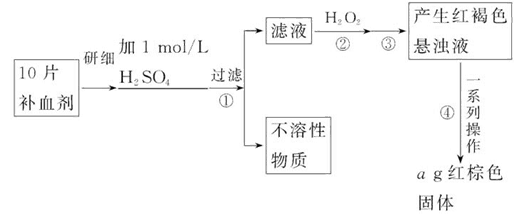
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cu��Fe3����Fe | B��Fe2����Fe3����Fe |
| C��Cu��Cu2����Fe | D��Cu��Fe2����Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ӧ��Ĺ��������л�����̼ |
| B����Ӧ��Ĺ�������������Ϊ13.6 g |
| C����Ӧ��Ĺ�������������������ʵ���Ϊ0.05 mol |
| D����Ӧ��Ĺ��������е���Cu������Ϊ12.8 g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com