
 A”¢B”¢C”¢D”¢E”¢F¶¼ŹĒÖÜĘŚ±ķÖŠĒ°ĖÄÖÜĘŚµÄŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó£¬ĘäÖŠA”¢B”¢C”¢D”¢EĪŖ²»Ķ¬Ö÷×åµÄŌŖĖŲ£®A”¢CµÄ×īĶā²ćµē×ÓŹż¶¼ŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬BµÄµēøŗŠŌ“óÓŚC£¬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģEµÄŃęÉ«·“Ó¦ĪŖ×ĻÉ«£¬FµÄ»łĢ¬Ō×ÓÖŠÓŠ4øöĪ“³É¶Ōµē×Ó£®
A”¢B”¢C”¢D”¢E”¢F¶¼ŹĒÖÜĘŚ±ķÖŠĒ°ĖÄÖÜĘŚµÄŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó£¬ĘäÖŠA”¢B”¢C”¢D”¢EĪŖ²»Ķ¬Ö÷×åµÄŌŖĖŲ£®A”¢CµÄ×īĶā²ćµē×ÓŹż¶¼ŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬BµÄµēøŗŠŌ“óÓŚC£¬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģEµÄŃęÉ«·“Ó¦ĪŖ×ĻÉ«£¬FµÄ»łĢ¬Ō×ÓÖŠÓŠ4øöĪ“³É¶Ōµē×Ó£® £®
£®·ÖĪö A”¢B”¢C”¢D”¢E”¢F¶¼ŹĒÖÜĘŚ±ķÖŠĒ°ĖÄÖÜĘŚµÄŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó£¬ĘäÖŠA”¢B”¢C”¢D”¢EĪŖ²»Ķ¬Ö÷×åµÄŌŖĖŲ£»A”¢CµÄ×īĶā²ćµē×ÓŹż¶¼ŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬ŌņAŹĒCŌŖĖŲ£¬CŹĒSŌŖĖŲ£¬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģEµÄŃęÉ«·“Ó¦ĪŖ×ĻÉ«£¬EŹĒKŌŖĖŲ£¬DµÄŌ×ÓŠņŹż½éÓŚSÓėKŌŖĖŲÖ®¼ä£¬¹ŹDĪŖClŌŖĖŲ£¬BµÄµēøŗŠŌ“óÓŚC£¬ĒŅBµÄŌ×ÓŠņŹżŠ”ÓŚC£¬ŹōÓŚ²»Ķ¬Ö÷×壬ĖłŅŌBŹĒNŌŖĖŲ£»FĪ»ÓŚµŚĖÄÖÜĘŚ£¬»łĢ¬Ō×ÓÖŠÓŠ4øöĪ“³É¶Ōµē×Ó£¬ĶāĪ§µē×ÓÅŲ¼ĪŖ3d64s2£¬ŌņFŹĒFeŌŖĖŲ£¬¾Ż“Ė½ųŠŠ½ā“š£®
½ā“š ½ā£ŗA”¢B”¢C”¢D”¢E”¢F¶¼ŹĒÖÜĘŚ±ķÖŠĒ°ĖÄÖÜĘŚµÄŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó£¬ĘäÖŠA”¢B”¢C”¢D”¢EĪŖ²»Ķ¬Ö÷×åµÄŌŖĖŲ£»A”¢CµÄ×īĶā²ćµē×ÓŹż¶¼ŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬ŌņAŹĒCŌŖĖŲ£¬CŹĒSŌŖĖŲ£¬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģEµÄŃęÉ«·“Ó¦ĪŖ×ĻÉ«£¬EŹĒKŌŖĖŲ£¬DµÄŌ×ÓŠņŹż½éÓŚSÓėKŌŖĖŲÖ®¼ä£¬¹ŹDĪŖClŌŖĖŲ£¬BµÄµēøŗŠŌ“óÓŚC£¬ĒŅBµÄŌ×ÓŠņŹżŠ”ÓŚC£¬ŹōÓŚ²»Ķ¬Ö÷×壬ĖłŅŌBŹĒNŌŖĖŲ£»FĪ»ÓŚµŚĖÄÖÜĘŚ£¬»łĢ¬Ō×ÓÖŠÓŠ4øöĪ“³É¶Ōµē×Ó£¬ĶāĪ§µē×ÓÅŲ¼ĪŖ3d64s2£¬ŌņFŹĒFeŌŖĖŲ£®
£Ø1£©»łĢ¬µÄFe3+ŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ£ŗ1s22s22p63s23p63d6 »ņ[Ar]3d6£¬¹Ź“š°øĪŖ£ŗ1s22s22p63s23p63d6 »ņ[Ar]3d6£»
£Ø2£©BµÄĘųĢ¬Ēā»ÆĪļĪŖNH3£¬A”¢CµÄĘųĢ¬Ēā»ÆĪļ·Ö±šĪŖCH4”¢H2S£¬NH3ÓėH2O·Ö×Ó¼ä“ęŌŚĒā¼ü£¬¶ųCH4”¢H2S²»ÄÜÓėĖ®·Ö×ÓŠĪ³ÉĒā¼ü£¬¹ŹNH3ŌŚĖ®ÖŠµÄČܽā¶ČŌ¶“óÓŚCH4”¢H2S£¬
¹Ź“š°øĪŖ£ŗNH3ÓėH2O·Ö×Ó¼ä“ęŌŚĒā¼ü£¬CH4”¢H2S·Ö×ÓÓėH2O·Ö×Ӽ䲻“ęŌŚĒā¼ü£»
£Ø3£©»ÆŗĻĪļFeCl3ŹĒ×ŲÉ«¹ĢĢ唢Ņ׳±½ā”¢100”ę×óÓŅŹ±Éż»Ŗ£¬ČŪ·ŠµćµĶ£¬ŹōÓŚ·Ö×Ó¾§Ģ壬»ÆŗĻĪļKSCNÖŠµÄŅõĄė×ÓÓėCS2»„ĪŖµČµē×ÓĢ壬øĆŅõĄė×ӵĵē×ÓŹ½ŹĒ £¬
£¬
¹Ź“š°øĪŖ£ŗ·Ö×Ó¾§Ģ壻 £»
£»
£Ø4£©øĆ$\frac{1}{8}$¾§°ūÖŠŗ¬ÓŠFe2+£ŗ4”Į$\frac{1}{8}$=$\frac{1}{2}$£»ŗ¬Fe3+£ŗ4”Į$\frac{1}{8}$=$\frac{1}{2}$£»ŗ¬CN-£ŗ12”Į$\frac{1}{4}$=3£®øł¾Ż»ÆŗĻ¼Ū“śŹżŗĶĪŖ0µÄŌŌņ£¬øĆ$\frac{1}{8}$¾§°ūÖŠŗ¬ÓŠK+£ŗ3-£Ø$\frac{1}{2}$”Į2+$\frac{1}{2}$”Į3£©=$\frac{1}{2}$£¬ŌņŅ»øö¾§°ūÖŠK+µÄøöŹżĪŖ$\frac{1}{2}$”Į8=4£¬
¹Ź“š°øĪŖ£ŗ4£»
£Ø5£©FeCl3ÓėKSCNµĆµ½ŗ¬¶ąÖÖÅäŗĻĪļµÄŃŖŗģÉ«ČÜŅŗ£¬ĘäÖŠÅäĪ»ŹżĪŖ5µÄÅäŗĻĪļµÄ»ÆѧŹ½ŹĒ£ŗK2Fe£ØSCN£©5£¬¹Ź“š°øĪŖ£ŗK2Fe£ØSCN£©5£®
µćĘĄ ±¾Ģāæ¼²éĪ»ÖĆ”¢½į¹¹ÓėŠŌÖŹ¹ŲĻµµÄ×ŪŗĻÓ¦ÓĆ£¬Éę¼°ŗĖĶāµē×ÓÅŲ¼”¢Ēā¼ü”¢¾§ĢåĄąŠĶÓėŠŌÖŹ”¢µČµē×ÓĢ唢ÅäŗĻĪļ”¢¾§°ū¼ĘĖćµČ£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·Ō×Ó½į¹¹ÓėŌŖĖŲÖÜĘŚĀÉ”¢ŌŖĖŲÖÜĘŚ±ķµÄ¹ŲĻµĪŖ½ā“š¹Ų¼ü£¬£Ø4£©ÖŠ×¢ŅāĄūÓĆ¾łĢƷؽųŠŠ¼ĘĖć£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
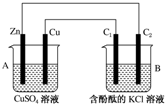 °“ČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃ飬²¢»Ų“šĻĀĮŠĪŹĢā£ŗ
°“ČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃ飬²¢»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
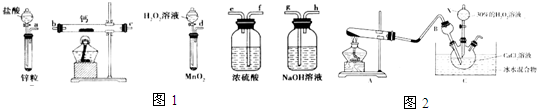
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ģś±ķĆę¶ĘĶ£¬±ķĆęÓŠ»®ĖšŹ±£¬Ņ²ÄÜ×čÖ¹Ģś±»Ńõ»Æ | |
| B£® | ŌŚæÕĘųÖŠ½šŹōĀĮ±ķĆęŃøĖŁ±»Ńõ»ÆŠĪ³É±£»¤Ä¤ | |
| C£® | ĢśĖæÓėÅØĮņĖįŌŚ³£ĪĀĻĀ¶Ū»Æ | |
| D£® | ŌŚĢśÓėĻ”H2SO4·“Ó¦Ź±£¬¼Ó¼øµĪCuSO4ČÜŅŗ£¬æɼÓæģH2µÄ²śÉś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
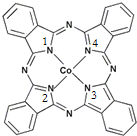 īÜ£ØCo£©ŹĒČĖĢå±ŲŠčµÄĪ¢ĮæŌŖĖŲ£®ŗ¬īÜ»ÆŗĻĪļ×÷ĪŖŃÕĮĻ£¬¾ßÓŠÓĘ¾ĆµÄĄśŹ·£¬ŌŚ»śŠµÖĘŌģ”¢“ÅŠŌ²ÄĮĻµČĮģÓņŅ²¾ßÓŠ¹ć·ŗµÄÓ¦ÓĆ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
īÜ£ØCo£©ŹĒČĖĢå±ŲŠčµÄĪ¢ĮæŌŖĖŲ£®ŗ¬īÜ»ÆŗĻĪļ×÷ĪŖŃÕĮĻ£¬¾ßÓŠÓĘ¾ĆµÄĄśŹ·£¬ŌŚ»śŠµÖĘŌģ”¢“ÅŠŌ²ÄĮĻµČĮģÓņŅ²¾ßÓŠ¹ć·ŗµÄÓ¦ÓĆ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ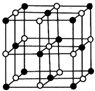 £¬ŌŚøĆ¾§ĢåÖŠÓėŅ»øöīÜŌ×ÓµČ¾ąĄėĒŅ×ī½üµÄīÜŌ×ÓÓŠ12øö£»Öž²Ø²ÄĮĻæĘѧ¹ś¼ŅŹµŃéŹŅŅ»øöæĘŃŠŠ”×é·¢ĻÖĮĖŌŚ 5K ĻĀ³ŹĻÖ³¬µ¼ŠŌµÄ¾§Ģ壬øĆ¾§Ģå¾ßÓŠCoO2µÄ²ćד½į¹¹£ØČēĻĀĶ¼ĖłŹ¾£¬Š”Ēņ±ķŹ¾CoŌ×Ó£¬“óĒņ±ķŹ¾OŌ×Ó£©£®ĻĀĮŠÓĆ“ÖĻß»³öµÄÖŲø“½į¹¹µ„ŌŖŹ¾ŅāĶ¼²»ÄÜĆčŹöCoO2µÄ»Æѧ×é³ÉµÄŹĒD£®
£¬ŌŚøĆ¾§ĢåÖŠÓėŅ»øöīÜŌ×ÓµČ¾ąĄėĒŅ×ī½üµÄīÜŌ×ÓÓŠ12øö£»Öž²Ø²ÄĮĻæĘѧ¹ś¼ŅŹµŃéŹŅŅ»øöæĘŃŠŠ”×é·¢ĻÖĮĖŌŚ 5K ĻĀ³ŹĻÖ³¬µ¼ŠŌµÄ¾§Ģ壬øĆ¾§Ģå¾ßÓŠCoO2µÄ²ćד½į¹¹£ØČēĻĀĶ¼ĖłŹ¾£¬Š”Ēņ±ķŹ¾CoŌ×Ó£¬“óĒņ±ķŹ¾OŌ×Ó£©£®ĻĀĮŠÓĆ“ÖĻß»³öµÄÖŲø“½į¹¹µ„ŌŖŹ¾ŅāĶ¼²»ÄÜĆčŹöCoO2µÄ»Æѧ×é³ÉµÄŹĒD£®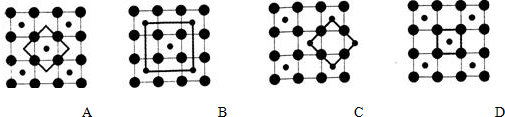
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µŖŌ×ÓµÄÖŹĮæ¾ĶŹĒµŖµÄĻą¶ŌŌ×ÓÖŹĮæ | |
| B£® | ŗ¤ĘųµÄĦ¶ūÖŹĮæŹĒ8g•mol-1 | |
| C£® | 1molH2SO4ÖŠŗ¬1molH2 | |
| D£® | 1molH2OµÄÖŹĮæŹĒ18g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
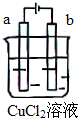
| A£® | µē¼«a±ķĆę³ŹŗģÉ« | |
| B£® | µē¼«b±ķĆęÓŠĘųÅŻÉś³É | |
| C£® | µē×ÓÓɵēŌ“µÄøŗ¼«ŃŲµ¼ĻßĮ÷Ļņµē¼«b | |
| D£® | øĆ×°ÖĆÄÜĮæ×Ŗ»ÆŠĪŹ½ĪŖ»ÆѧÄÜ×Ŗ»ÆĪŖµēÄÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
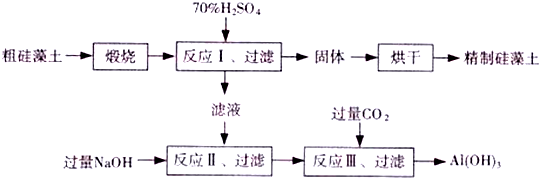
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £©Ņ»ÖÖÖĪĮĘŠÄŃŖ¹ÜŗĶøßŃŖŃ¹µÄŅ©Īļ£¬æÉÓÉ»ÆŗĻĪļB£Ø
£©Ņ»ÖÖÖĪĮĘŠÄŃŖ¹ÜŗĶøßŃŖŃ¹µÄŅ©Īļ£¬æÉÓÉ»ÆŗĻĪļB£Ø £©ĶعżŅŌĻĀĀ·ĻßŗĻ³É£ŗ
£©ĶعżŅŌĻĀĀ·ĻßŗĻ³É£ŗ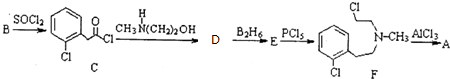
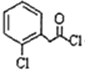 +CH3NH£ØCH2£©2OH”ś
+CH3NH£ØCH2£©2OH”ś +HCl£®
+HCl£® £®ÓÉFÉś³ÉAµÄ·“Ó¦ĄąŠĶĪŖČ”“ś·“Ó¦£®
£®ÓÉFÉś³ÉAµÄ·“Ó¦ĄąŠĶĪŖČ”“ś·“Ó¦£® »ņ
»ņ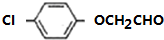 £®
£®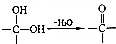 £®Š“³öŅŌ
£®Š“³öŅŌ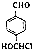 ĪŖŌĮĻÖʱø»ÆŗĻĪļ
ĪŖŌĮĻÖʱø»ÆŗĻĪļ µÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼£®
µÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼£® £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com