
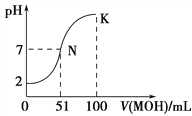
”¾ĢāÄæ”æ³£ĪĀĻĀ£¬Ļņ100 mL 0.01 mol”¤L£1HAČÜŅŗÖŠÖšµĪ¼ÓČė0.02 mol”¤L£1MOHČÜŅŗ£¬Ķ¼ÖŠĖłŹ¾ĒśĻß±ķŹ¾»ģŗĻČÜŅŗµÄpH±ä»ÆĒéæö(ČÜŅŗĢå»ż±ä»ÆŗöĀŌ²»¼Ę)”£ĻĀĮŠĖµ·ØÖŠ£¬²»ÕżČ·µÄŹĒ
A. HAĪŖŅ»ŌŖĒæĖį
B. MOHĪŖŅ»ŌŖČõ¼ī
C. NµćĖ®µÄµēĄė³Ģ¶ČŠ”ÓŚKµćĖ®µÄµēĄė³Ģ¶Č
D. ČōKµć¶ŌÓ¦ČÜŅŗµÄpH£½10£¬ŌņÓŠc(MOH)£«c(OH£)£c(H£«)£½0.005 mol”¤L£1
”¾“š°ø”æC
”¾½āĪö”æŹŌĢā·ÖĪö£ŗÓÉĶ¼Ļń·ÖĪöÖŖ¼ÓČė¼īµÄĢå»żĪŖ51mLŹ±ČÜŅŗ³ŹÖŠŠŌ£¬ĖµĆ÷¶žÕß²»¶¼ŹĒĒæµē½āÖŹ£¬Čō¼īĪŖĒæ¼īŌņ¼ÓČė¼ī50mLŹ±Ē”ŗĆĶźČ«·“Ó¦£¬“ĖŹ±ĪŖĒæ¼īČõĖįŃĪ£¬ČÜŅŗÓ¦³Ź¼īŠŌ£¬ÓėĶ¼Ļń²»·ū£¬ĖłŅŌĖįÓ¦ĪŖĒæĖį£¬¼īĪŖČõ¼ī”£A”¢BÕżČ·£»C”¢NµćŹ±£¬ČÜŅŗ³ŹÖŠŠŌ£¬ĒāĄė×ÓÅØ¶ČµČÓŚĒāŃõøłĄė×ÓÅضČ=1”Į10-7mol/L£¬¶ųKµćŹ±¼ī¹żĮ棬ŅÖÖĘĖ®µÄµēĄė£¬ĖłŅŌNµćĖ®µÄµēĄė³Ģ¶Č“óÓŚKµćĖ®µÄµēĄė³Ģ¶Č£¬“ķĪó£»D”¢ŌŚKµćŹ±¼ÓČė¼īµÄĢå»żĪŖ100mL£¬“ĖŹ±ČÜŅŗŹĒMOHÓėMAµČÅØ¶ČµÄ»ģŗĻŅŗĒŅÅØ¶Č¶¼ĪŖ0.005mol/L,øł¾ŻĪļĮĻŹŲŗćµĆc(MOH)+c(M+)="0.01" mol/L,øł¾ŻµēŗÉŹŲŗćµĆc(M+)+c(H+)=c(A-)+c(OH-),¶žŹ½½įŗĻµĆ
c(MOH)+ c(OH-)- c(H+)="0.01-" c(A-)=0.01-0.005=0.005mol/L£¬ÕżČ·£¬“š°øŃ”C”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æNA“ś±ķ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A. ³£ĪĀ³£Ń¹ĻĀ£¬124 g P4ÖŠĖłŗ¬P”ŖP¼üŹżÄæĪŖ4NA
B. 100 mL 1mol”¤L1FeCl3ČÜŅŗÖŠĖłŗ¬Fe3+µÄŹżÄæĪŖ0.1NA
C. ±ź×¼×“æöĻĀ£¬11.2 L¼×ĶéŗĶŅŅĻ©»ģŗĻĪļÖŠŗ¬ĒāŌ×ÓŹżÄæĪŖ2NA
D. ĆܱÕČŻĘ÷ÖŠ£¬2 mol SO2ŗĶ1 mol O2“߻Ʒ“Ó¦ŗó·Ö×Ó×ÜŹżĪŖ2NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĪļÖŹÖŠ£¬¼ČÄÜŅņ·¢Éś»Æѧ·“Ó¦¶ųŹ¹äåĖ®ĶŹÉ«£¬ÓÖÄÜŹ¹øßĆĢĖį¼ŲĖįŠŌČÜŅŗĶŹÉ«µÄŹĒ( )
¢ŁCH3CH2CH2CH3 ¢ŚCH3CH2CH===CH2

A£®¢Ł¢Ś¢Ū¢Ü B£®¢Ś¢Ū¢Ü
C£®¢Ś¢Ü D£®Ö»ÓŠ¢Ś
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©ŌŚNaClČÜŅŗÖŠµĪ¼ÓAgNO3ČÜŅŗ£¬ĻÖĻóĪŖ_____________________________ £¬ Ąė×Ó·½³ĢŹ½ĪŖ:_________________________________________________
£Ø2£©ŌŚCH3CH2CH2ClÖŠµĪ¼ÓAgNO3ČÜŅŗ£¬ĻÖĻóĪŖ_____________________________£¬ŌŅņŹĒ__________________________
£Ø3£©ČōĻČ½«CH3CH2CH2ClÓėNaOHČÜŅŗ¹²ČČ£¬Č»ŗóÓĆĻõĖįĖį»Æ£¬ŌŁµĪ¼ÓAgNO3ČÜŅŗ£¬ĻÖĻóĪŖ_______________________________________________________£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ:________________________£¬______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA”¢B”¢C”¢W¾łĪŖ֊ѧ³£¼ūµÄĪļÖŹ£¬ĖüĆĒÖ®¼äÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£ØĘäĖū²śĪļ¼°·“Ó¦Ģõ¼žŅŃĀŌČ„£¬·“Ó¦æÉŅŌŌŚĖ®ČÜŅŗÖŠ½ųŠŠ£©”£

£Ø1£©ČōA”¢B”¢CČżÖÖĪļÖŹµÄŃęÉ«·“Ó¦¾łĪŖ»ĘÉ«£¬AĖ׳ĘæĮŠŌÄĘ£¬WĪŖĪŽÉ«ĪŽĪ¶ĘųĢ壬CŹÜČČ·Ö½āæÉ×Ŗ»ÆĪŖB”£
¢ŁĻņBČÜŅŗÖŠĶØČėWÉś³ÉCµÄĄė×Ó·½³ĢŹ½ĪŖ_______________”£
¢ŚAČÜŅŗÓėCČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________”£
£Ø2£©ČōA”¢B”¢C”¢WĖÄÖÖĪļÖŹ¾łĪŖĘųĢ壬ĘäÖŠA”¢WĪŖµ„ÖŹ£¬CµÄĦ¶ūÖŹĮæĪŖ46g”¤mol-1.
¢ŁBµÄ»ÆѧŹ½ĪŖ________________”£
¢ŚŹµŃéŹŅÖĘČ”BŹ±£¬_____________£ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©ÓĆĻņÉĻÅÅæÕĘų·ØŹÕ¼ÆB”£
¢ŪCÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________________________”£
£Ø3£©ČōAĪŖµ»ĘÉ«¹ĢĢåµ„ÖŹ£¬WĪŖĘųĢåµ„ÖŹ£¬£Ā”¢£Ć¾łĪŖĖįŠŌŃõ»ÆĪļ”£
¢ŁÓÉBÉś³ÉCŹ±£¬ĆæÉś³É1molC£¬ĻūŗÄWµÄĪļÖŹµÄĮæĪŖ_________________”£
¢ŚCČÜÓŚĖ®ŠĪ³É»ÆŗĻĪļD£¬ŌŚ¼ÓČȵÄĒéæöĻĀ£¬DµÄÅØČÜŅŗÓėA·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ25”ꏱ£¬5ÖÖŅųŃĪµÄČܶȻż³£Źż£ØKsp£©·Ö±šŹĒ£ŗ
AgCl | Ag2SO4 | Ag2S | AgBr | AgI |
1.8”Į10-10 | 1.4”Į10-5 | 6.3”Į10-50 | 7.7”Į10-13 | 8.51”Į10-16 |
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A. ĀČ»ÆŅų”¢äå»ÆŅųŗĶµā»ÆŅųµÄČܽā¶ČŅĄ“ĪŌö“ó
B. ½«ĮņĖįŅųČܽāÓŚĖ®ŗó£¬ĻņĘäÖŠ¼ÓČėÉŁĮæĮņ»ÆÄĘ¹ĢĢ壬²»ÄܵƵ½ŗŚÉ«³Įµķ
C. ŌŚ5mL1.5”Į10-5molL-1µÄNaClČÜŅŗÖŠ£¬¼ÓČė1µĪ£Ø1mLŌ¼20µĪ£©1.0”Į10-3molL-1µÄAgNO3ČÜŅŗ£¬²»ÄܹŪ²ģµ½°×É«³Įµķ
D. ŌŚÉÕ±ÖŠ·ÅČė6.24 gĮņĖįŅų¹ĢĢ壬¼Ó200 g Ė®£¬¾³ä·ÖČܽāŗó£¬ĖłµĆ±„ŗĶČÜŅŗµÄĢå»żĪŖ200 mL£¬ČÜŅŗÖŠAg + µÄĪļÖŹµÄĮæÅضČĪŖ0.2molL-1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æµŖŃ»·ŹĒÉśĢ¬ĻµĶ³ĪļÖŹŃ»·µÄÖŲŅŖ×é³É²æ·Ö£¬ČĖĄą»ī¶ÆÓ°ĻģĮĖµŖŃ»·ÖŠµÄĪļÖŹ×Ŗ»Æ£¬ČēÓŅĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÖŠ£¬²»ÕżČ·µÄŹĒ

A. ¹¤ŅµŗĻ³É°±ŹōÓŚČĖ¹¤¹ĢµŖ¹ż³Ģ
B. Ļõ»Æ¹ż³ĢÖŠµŖŌŖĖŲ±»»¹Ō
C. ŗ¬µŖĪŽ»śĪļÓėŗ¬µŖÓŠ»ś»ÆŗĻĪļæÉĻą»„×Ŗ»Æ
D. ·“Ļõ»Æ¹ż³ĢÓŠÖśÓŚĪȶØN2ŌŚ“óĘųÖŠµÄŗ¬Įæ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA”¢B”¢C”¢DŹĒ֊ѧ»Æѧ֊µÄ³£¼ūĪļÖŹ£¬ø÷ĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ
![]()
(1)ČōAŹĒ»ĘÉ«¹ĢĢ壬BŹĒŠĪ³ÉĖįÓźµÄÖ÷ŅŖĪļÖŹÖ®Ņ»”£
¢Ł»³öAµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼____________”£
¢Ś Š“³öDµÄÅØČÜŅŗĖł¾ßÓŠµÄĢŲŹāŠŌÖŹ________________(Į½ÖÖ¼“æÉ)”£
(2)Čō“Ė·“Ó¦Į÷³ĢÄܽāŹĶÅ©Ńč”°Ą×Óź·¢×ƼŚ”±µÄ»ÆѧŌĄķ£¬ŌņĻĀĮŠŠšŹöÕżČ·µÄŹĒ________”£
a.AµÄ»ÆѧŠŌÖŹ½ĻĪŖĪČ¶Ø b.B”¢C¾łĪŖĖįŠŌŃõ»ÆĪļ
c.Į÷³ĢÖŠµÄ·“Ó¦¾łŹōŃõ»Æ»¹Ō·“Ó¦ d.æÉÓĆDÓė½šŹō·“Ó¦ÖĘČ”ĒāĘų
(3)ČōA”¢B”¢C”¢DµÄŃęÉ«·“Ó¦¾łĪŖ»ĘÉ«£¬CĪŖµ»ĘÉ«¹ĢĢ壬AŌŚ¼ÓČČĢõ¼žĻĀæÉÖ±½Ó×Ŗ»ÆĪŖC£¬Ōņ C”śDµÄ»Æѧ·½³ĢŹ½ŹĒ__________”£
(4)ČōAŹĒ»ÆŗĻĪļ£¬ĒŅ“ĖĮ÷³Ģ±ķŹ¾¹¤ŅµÖĘĻõĖįµÄĪļÖŹ×Ŗ»Æ¹ŲĻµ£¬ŌņA”śBµÄ·“Ó¦·½³ĢŹ½ĪŖ_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¶ĢÖÜĘŚŌŖĖŲ¼×”¢ŅŅ”¢±ū”¢¶””¢Īģ”¢¼ŗ”¢øżŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĶ¼£Ø¼×²»Ņ»¶ØŌŚ¶””¢øżµÄĮ¬ĻßÉĻ£©£¬Īģ”¢¼ŗ·Ö±šŹĒæÕĘų”¢µŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ”£ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ

A. ¼×Ņ»¶ØŹĒ½šŹōŌŖĖŲ
B. ĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌ£ŗøż>¼ŗ>Īģ
C. ŅŅ”¢±ū”¢¶”µÄ×īøß¼ŪŃõ»ÆĪļĖ®»ÆĪļæÉŅŌŅŌĻą»„·“Ó¦
D. øżµÄ×īøß¼ŪŃõ»ÆĪļĖ®»ÆĪļĖįŠŌ×īĒæ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com