
ij��ɫ��Һ���ܺ����������εļ��֣�(A)�Ȼ��� (B)���� (C)�������� (D)��������� (E)������ (F)̼���ƣ������Һ�м���������ϡ���ᣬ�гʻ�ɫ�ij���������ͬʱ������������������г�������ζ����ʹ�����ʯ��ˮ����ǣ�����ʹƷ����Һ��ɫ����������ʵ������ش��������⣮
����
(1)����ʹƷ����Һ��ɫ��˵���������в���________(�����ʽ)
(2)����ɫ��Һ���������ļ����Σ���д��ȫ�����ܵ����(��д��Ӧ����ĸ���ɲ�������Ҳ�ɲ���)
��һ�������________���ڶ��������________________��
�����������________�������������________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�
ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�| ������ | CO32-��SiO32-��AlO2-��Cl- |
| ������ | Al3+��Cu2+��Mg2+��NH4+��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �� I��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�
�� I��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�| ������ | CO32-��SiO32-��AlO2-��Cl- |
| ������ | Al3+��Cu2+��Mg2+��NH4+��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ������ѧ�ڵڶ����¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��1����Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ��������������֮������֪��Ԫ�ؾ����������ʣ�
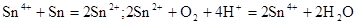 ��
��




 ��
��
�Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��_______________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������__________���ѧʽ����
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü��ѡ��________��
��2��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
|
������ |
|
|
������ |
|
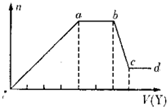
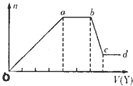
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ�����n��������Լ�Y�����V���Ĺ�ϵ��ͼ��ʾ��

����Y�����ᣬ����Һ�к��еĽ�����������_________��ab�η�����Ӧ�����ӷ���ʽΪ_______________��ͼ��oa�βμӷ�Ӧ�������ӵ����ʵ���֮��Ϊ___________________��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ____________________��
�����������ӵ�ˮ�⣬����H����OH����Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ_____________________________________________������������ǰ���������ں���ǰ���ͼ��ں��˳�����У���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡ�߶��ڶ�ѧ�����п��Ի�ѧ���� ���ͣ�ѡ����
���и������¿��ܹ������������
A��ij��ɫ��Һ�У�NH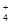 ��Na+��Cl����MnO
��Na+��Cl����MnO
B���ں��д���I�����ӵ���Һ�У�Cl¯��Fe3+��Al3+��Cu2+
C����c(H+)=1��10��13mol��L��1����Һ�У�NH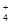 ��Al3+��SO
��Al3+��SO ��CO
��CO
D���ڼ���Al�ܷų�����H2����Һ�У�NH4+ ��SO42¯ ��C1¯��Na+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ����9���¿���ѧ���� ���ͣ�ʵ����
�� 14 �֣�ij��ɫ����Һ���ܺ����������ӣ�K+��Fe3+��Cu2+��Ba2+��AlO��NO3����CO32����SO32����SO42����Br����I����Cl����ȡ����Һ�����²������ʵ�顣
��1����������±������е�ʵ�������ش�������й����⣺
|
��ʵ���� |
��ش����� |
|
��ȡԭ��Һ�������μ�������ˮ���������������ˣ���Һ��CCl4����CCl4��δ��ɫ�� |
�ٸò�ʵ��˵����Һ�в����� ����_________________�� |
|
��ȡԭ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ�� |
�ڸò�ʵ�鷢����Ӧ�����ӷ���ʽΪ _________________�� |
|
��ȡԭ��Һ�������������ữ���백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ�� |
�۲�����ɫ���������ӷ���ʽΪ _________________�� |
|
��ȡԭ��Һ�������μ�2�����Ը��������Һ����ɫ������ȥ�� |
�ܸò�ʵ��˵����Һ�д��� ���� _________________�� |
|
��ȡԭ��Һ������������Ba��NO3��2��Һ������ɫ���������ˣ���ϴ�Ӻ�ij����м����������ᣬ���������ܽ⣬����ɫ��������� |
��δ�ܽ�İ�ɫ������ ��������ͨ��Ʒ����Һ�������� �� |
|
��ȡ�����������Һ����HNO3�ữ���ټ�AgNO3��Һ����Һ��������ɫ������ |
�ò����Ƿ������� ����С����ޡ����� |
��2�����ϣ�����Һ��pH ���������������������7������Һ��һ�����ڵ��������� ������ȷ���Ƿ���ڵ������� ������ȡԭ��Һ�������Ƿ���ڣ��Ƿ�Ҫʹ���Լ�������Ҫʹ���Լ�����ʵ�鷽���� ����Ҫʹ���Լ���ѡ�õ��Լ��ǣ���ʹ�õ��Ⱥ�˳������д����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com