
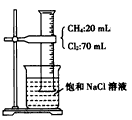
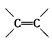
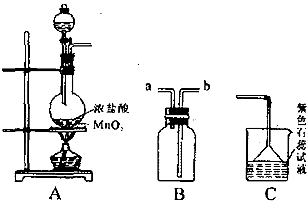
 �Ļ�������CH2=CH2һ������һ�������¿ɾۺϳɸ߷��ӻ�����ٹ㷺����ũ�ñ�Ĥ�ľ�����ϩ���ϣ�����
�Ļ�������CH2=CH2һ������һ�������¿ɾۺϳɸ߷��ӻ�����ٹ㷺����ũ�ñ�Ĥ�ľ�����ϩ���ϣ�����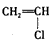 �ۺ϶��ɵģ���ۺϵĻ�ѧ����ʽ��__________________________�� �ڵ�����װ�д���ʹ�õ���ĭ���ϵ���Ҫ�ɷ��Ǿ۱���ϩ
�ۺ϶��ɵģ���ۺϵĻ�ѧ����ʽ��__________________________�� �ڵ�����װ�д���ʹ�õ���ĭ���ϵ���Ҫ�ɷ��Ǿ۱���ϩ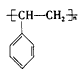 ������_____________________��д�ṹ��ʽ���ۺ϶��ɵġ�
������_____________________��д�ṹ��ʽ���ۺ϶��ɵġ�  �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?�㶫�����ù��ܺ�������ɽ�CO2��H2O��g��ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I��II��III�������£�CH4���������ʱ��ı仯��ͼ��ʾ��
��2011?�㶫�����ù��ܺ�������ɽ�CO2��H2O��g��ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I��II��III�������£�CH4���������ʱ��ı仯��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ͨ��ú��������Һ����ʹ̼���仯������Թ㷺Ӧ�ã�
ͨ��ú��������Һ����ʹ̼���仯������Թ㷺Ӧ�ã�| t/min | 0 | 1 | 2 | 3 | 4 |
| n��H2O��/mol | 0.600 | 0.520 | 0.450 | 0.350 | 0.350 |
| n��CO��/mol | 0.400 | 0.320 | 0.250 | 0.150 | 0.150 |
| ����/Ҷ���� |
| ||
| �� |
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��̩�ݶ��и߶���ѧ�����п��Ի�ѧ��ѡ�ޣ��Ծ��������棩 ���ͣ������
��14�֣����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I,II,III�������£�CH4���������ʱ��ı仯��ͼ��ʾ��

��1����0-30Сʱ�ڣ�CH4��ƽ����������v����v����v���Ӵ�С��˳��Ϊ ��
��Ӧ��ʼ���12Сʱ�ڣ��ڵ� �ִ����������£��ռ���CH4��ࡣ
��2��������CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ��CH4(g)+H2O(g) CO(g)+3H2(g),�÷�Ӧ�Ħ�H=+206 kJ•mol-1�������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬ƽ�ⳣ��K=27����ʱ���CO�����ʵ���Ϊ0.10mol����CH4��ƽ��ת���ʣ�������������λ��Ч���֣�
CO(g)+3H2(g),�÷�Ӧ�Ħ�H=+206 kJ•mol-1�������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬ƽ�ⳣ��K=27����ʱ���CO�����ʵ���Ϊ0.10mol����CH4��ƽ��ת���ʣ�������������λ��Ч���֣�
��3����֪��CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H=��802kJ•mol-1
д����CO2����CO���Ȼ�ѧ����ʽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com