
 N2£Øg£©+ CO2£Øg£©£»”÷H= Q kJ?mol-1”£ŌŚT1”ꏱ£¬·“Ó¦½ųŠŠµ½²»Ķ¬Ź±¼ä²āµĆø÷ĪļÖŹµÄÅضČČēĻĀ£ŗ
N2£Øg£©+ CO2£Øg£©£»”÷H= Q kJ?mol-1”£ŌŚT1”ꏱ£¬·“Ó¦½ųŠŠµ½²»Ķ¬Ź±¼ä²āµĆø÷ĪļÖŹµÄÅضČČēĻĀ£ŗ| Ź±¼ä£Ømin£© ÅØ¶Č£Ømol/L£© | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1£®00 | 0£®68 | 0£®50 | 0£®50 | 0£®60 | 0£®60 |
| N2 | 0 | 0£®16 | 0£®25 | 0£®25 | 0£®30 | 0£®30 |
| CO2 | 0 | 0£®16 | 0£®25 | 0£®25 | 0£®30 | 0£®30 |
 (1·Ö)
(1·Ö)

 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 H2(g)+CO2(g) ¦¤H£½£41kJ”¤mol£1
H2(g)+CO2(g) ¦¤H£½£41kJ”¤mol£1 CH4(g) ¦¤H£½£73kJ”¤mol£1
CH4(g) ¦¤H£½£73kJ”¤mol£1 C(s)+CO2(g) ¦¤H£½£171kJ”¤mol£1
C(s)+CO2(g) ¦¤H£½£171kJ”¤mol£1 CH3OCH3(g) + 3H2O(g)”£ŅŃÖŖŅ»¶ØĢõ¼žĻĀ£¬øĆ·“Ó¦ÖŠCO2µÄĘ½ŗā×Ŗ»ÆĀŹĖęĪĀ¶Č”¢Ķ¶ĮĻ±Č[n(H2) / n(CO2)]µÄ±ä»ÆĒśĻßČēĻĀ×óĶ¼£ŗ
CH3OCH3(g) + 3H2O(g)”£ŅŃÖŖŅ»¶ØĢõ¼žĻĀ£¬øĆ·“Ó¦ÖŠCO2µÄĘ½ŗā×Ŗ»ÆĀŹĖęĪĀ¶Č”¢Ķ¶ĮĻ±Č[n(H2) / n(CO2)]µÄ±ä»ÆĒśĻßČēĻĀ×óĶ¼£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 CH3OH(g) ¦¤H
CH3OH(g) ¦¤H
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®392£®93 Kj | B£®2 489£®42 kJ |
| C£®784£®92 kJ | D£®3 274£®3 kJ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 O2(g)===H2O(l) ¦¤H3£½£285.84 kJ”¤mol£1
O2(g)===H2O(l) ¦¤H3£½£285.84 kJ”¤mol£1²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

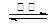 4CO(g) + BaS(s) ¦¤H1 = +571£®2 kJ”¤mol-1 ¢Ł
4CO(g) + BaS(s) ¦¤H1 = +571£®2 kJ”¤mol-1 ¢Ł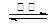 2CO2(g) + BaS(s) ¦¤H2= +226£®2 kJ”¤mol-1 ¢Ś
2CO2(g) + BaS(s) ¦¤H2= +226£®2 kJ”¤mol-1 ¢Ś| A£®Na2SČÜŅŗµÄpH±ČNaHSČÜŅŗpHŠ” |
| B£®Į½ČÜŅŗÖŠŗ¬ÓŠµÄĄė×ÓÖÖĄą²»Ķ¬ |
| C£®Į½ČÜŅŗÖŠµĪČėĶ¬Ģå»żĶ¬ÅØ¶ČµÄŃĪĖį£¬²śÉśĘųĢåĖŁĀŹĻąµČ |
| D£®Į½ČÜŅŗÖŠ¼ÓČėNaOH¹ĢĢ壬c(S2-)¶¼Ōö“ó |
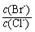 = ”£ ”¾ŅŃÖŖ£ŗKsp(AgBr)=5£®4”Į10-13£¬Ksp(AgCl)=2£®0”Į10-10”æ
= ”£ ”¾ŅŃÖŖ£ŗKsp(AgBr)=5£®4”Į10-13£¬Ksp(AgCl)=2£®0”Į10-10”æ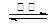 2CO(g)µÄ¦¤H =
2CO(g)µÄ¦¤H = ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 2NH3(g)£»¦¤H£½£92.4 kJ/mol
2NH3(g)£»¦¤H£½£92.4 kJ/mol
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com