���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT
1��T
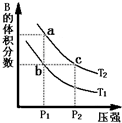
2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮
��1����������ͼ����������˵����ȷ����
BC
BC
������ĸ����
A��a��c����ķ�Ӧ���ʣ�a��c
B����״̬b��״̬a������ͨ�����ȵķ���
C��b��c����A�����ת�������
��2��������Ӧ���ܱ����������ݣ��н��У��ﵽƽ��״̬�ı�־��
BE
BE
������ĸ����
A����λʱ��������n mol A��ͬʱ�ֽ�2n molB
B���������������������ٸı�
C��v
����A��=2v
����B��
D�����������ܶȲ��ٷ����仯
E����������ѹǿ���ٷ����仯
��3����������Ӧ��ƽ��ʱ��B�����ƽ��Ũ��Ϊ0.1mol?L
-1��ͨ����С�����������ϵ��ѹǿ���¶ȱ��ֲ��䣩�����´�ƽ���B�����ƽ��Ũ��
��
��
0.1mol?L
-1�����������������=������
��4����100��ʱ����0.40mol��B�������2L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±������ݣ�
| ʱ�䣨s�� |
0 |
20 |
40 |
60 |
80 |
| n��B��/mol |
0.40 |
n1 |
0.26 |
n3 |
n4 |
| n��A��/mol |
0.00 |
0.05 |
n2 |
0.08 |
0.08 |
�������������£��ӷ�Ӧ��ʼ��40sʱ����A�����ʾ�ĸ÷�Ӧ��ƽ����Ӧ����Ϊ
0.000875mol/��L?s��
0.000875mol/��L?s��
��
���ϱ���n
3=
=
n
4�����������������=��������ӦA��g��?2B��g����100��ʱ��ƽ�ⳣ��K��ֵΪ
0.36
0.36
�������¶Ⱥ�Ӧ2B��g��?A��g����ƽ�ⳣ��K��ֵ
��С
��С
�����������С�����䡱����
��������ͬ����������������г������A���壬Ҫ�ﵽ����ͬ����ƽ��״̬��A�������ʼŨ��Ϊ
0.1
0.1
mol?L
-1��

![]()
![]()
![]()
![]() ��������0.5mol H2SO4��Ũ�����뺬1mol NaOH����Һ��ϣ��ų�����������57.3KJ
��������0.5mol H2SO4��Ũ�����뺬1mol NaOH����Һ��ϣ��ų�����������57.3KJ
 ���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮
���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮

