
ʳ�Ρ�̼���ƺ�̼�������������г��������Ρ���ش��������⡣
��1��̼�����Ƶ�ˮ��Һ��__________�ԡ�����ᡱ��������С�������ȥ̼���ƹ����л��е�����̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ_________________��
��2����������̼���ƺ�̼�����Ʒֱ����������ᷴӦʱ���ɵ�CO2����ǰ��________���ߣ��>������<����=������
��3�����κ����������ʣ���ҪΪCaCl2��MgCl2��Na2SO4�ȣ����ô�����ȡ����ѧ��������NaCl������Ϊ�ܽ⡢�ӹ���a���ӹ���NaOH���ӹ���b�����ˡ����������ᣬ�����ᾧ�õ�����ѧ��������NaCl���塣�Լ�a��b�ֱ���________(����ţ�
A.Na2CO3 BaCl2 B.BaCl2 Na2CO3 C.BaCl2 Na2SO4
��4����ҵ���õ�ⱥ��ʳ��ˮ�ķ��������������ռ
��ij��������������й©�¼���������Ա����NaOH��Һ�γ�ҺĻ����Χ������й©���������䷴Ӧԭ��_________________________________�������ӷ���ʽ��ʾ����
�ڹ�ҵ�Ͽ��ð��������������Ĺܵ��Ƿ�©������Ӧ����ʽ���£� �÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
�÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
��1���� 2NaHCO3 Na2CO3 + CO2��+ H2O��2���� ��3��B��4����Cl2 + 2OH-=Cl- + ClO- + H2O�����ȣ�3:2��
Na2CO3 + CO2��+ H2O��2���� ��3��B��4����Cl2 + 2OH-=Cl- + ClO- + H2O�����ȣ�3:2��
���������������1��̼�����Ƶ�ˮ��Һ�Լ��ԣ�̼���������ȷֽ�����̼���ơ�������̼��ˮ��̼���������ѷֽ⣬��ȥ̼���ƹ����л��е�����̼�����ƿ��ü��ȷ�����Ӧ�Ļ�ѧ����ʽΪ2NaHCO3 Na2CO3 + CO2��+ H2O����2��̼���Ƶ���Է�������Ϊ106��̼�����Ƶ���Է�������Ϊ84����������̼���ƺ�̼�����ƣ�̼���Ƶ����ʵ���С��̼�����Ƶ����ʵ���������̼�غ��֪���ֱ����������ᷴӦʱ���ɵ�CO2����ǰ��С�ں��ߣ���3�����κ����������ʣ���ҪΪCaCl2��MgCl2��Na2SO4�ȣ����ô�����ȡ����ѧ��������NaCl��Ϊ���������ӳ����������Լ�Ҫ�������������Լ����ں��������б����ȥ�������������Ƴ�ȥþ���ӣ����Ȼ�����ȥ���������̼���Ƴ�ȥ�����Ӽ������ı����ӣ�ʵ�鲽��Ϊ�ܽ⡢�ӹ���BaCl2���ӹ���NaOH���ӹ���Na2CO3�����ˡ����������ᣬ�����ᾧ�õ�����ѧ��������NaCl���塣ѡB����4��������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�����ӷ���ʽΪCl2 + 2OH-=Cl- + ClO- + H2O���ڹ�ҵ�Ͽ��ð��������������Ĺܵ��Ƿ�©�����÷�Ӧ�У���Ԫ�صĻ��ϼ۽��ͣ�����ԭ���������������������е�Ԫ�صĻ��ϼ����ߣ�����������������ԭ�����μӵİ�����ֻ��2�����Ӱ������������÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ3:2��
Na2CO3 + CO2��+ H2O����2��̼���Ƶ���Է�������Ϊ106��̼�����Ƶ���Է�������Ϊ84����������̼���ƺ�̼�����ƣ�̼���Ƶ����ʵ���С��̼�����Ƶ����ʵ���������̼�غ��֪���ֱ����������ᷴӦʱ���ɵ�CO2����ǰ��С�ں��ߣ���3�����κ����������ʣ���ҪΪCaCl2��MgCl2��Na2SO4�ȣ����ô�����ȡ����ѧ��������NaCl��Ϊ���������ӳ����������Լ�Ҫ�������������Լ����ں��������б����ȥ�������������Ƴ�ȥþ���ӣ����Ȼ�����ȥ���������̼���Ƴ�ȥ�����Ӽ������ı����ӣ�ʵ�鲽��Ϊ�ܽ⡢�ӹ���BaCl2���ӹ���NaOH���ӹ���Na2CO3�����ˡ����������ᣬ�����ᾧ�õ�����ѧ��������NaCl���塣ѡB����4��������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�����ӷ���ʽΪCl2 + 2OH-=Cl- + ClO- + H2O���ڹ�ҵ�Ͽ��ð��������������Ĺܵ��Ƿ�©�����÷�Ӧ�У���Ԫ�صĻ��ϼ۽��ͣ�����ԭ���������������������е�Ԫ�صĻ��ϼ����ߣ�����������������ԭ�����μӵİ�����ֻ��2�����Ӱ������������÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ3:2��
���㣺����̼���Ƽ�̼�����Ƶ����ʡ����ε��ᴿ��������ԭ��Ӧ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֡�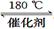
��1�����ü������ԭ���������֪��
��CH4 (g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g�� ��H ����574 kJ/mol
��CH4(g)��4NO(g���� 2N2(g)��CO2(g)��2H2O(g�� ��H ����1160 kJ/mol
��CH4 ��NO2 ��ԭΪN2 ���Ȼ�ѧ����ʽΪ�� ��
��2������NH3����ԭ�������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ: Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
��3������ClO2�������������ת����������: NO NO2
NO2 N2����֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O ��NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
N2����֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O ��NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
��4���û���̿��ԭ��������������йط�ӦΪ��C��s��+2NO��g�� N2 ��g��+CO2 ��g����H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2 ��g��+CO2 ��g����H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| Ũ��/mol?L-1/ ʱ��/min | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.040 | 0.030 | 0.030 |
| 30 | 0.040 | 0.030 | 0.030 |
| 40 | 0.032 | 0.034 | 0.017 |
| 50 | 0.032 | 0.034 | 0.017 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������(K2FeO4)��һ�����͡���Ч�������ɫˮ����������Cl2��O2��ClO2��KMnO4�����Ը�ǿ��������Ⱦ����ҵ�������Ƶø������ƣ�Ȼ���ڵ����£������������Һ�м���KOH�����ͣ�ʹ�������������
(1)�ɷ��Ʊ�������ص���Ҫ��ӦΪ��2FeSO4�� 6Na2O2=2Na2FeO4�� 2Na2O �� 2Na2SO4�� O2��
�ٸ÷�Ӧ�е���������________����ԭ����________��ÿ����1 mol Na2FeO4ת��________mol���ӡ�
�ڼ�Ҫ˵��K2FeO4��Ϊˮ������ʱ���������__________________________________
(2)ʪ���Ʊ��������(K2FeO4)�ķ�Ӧ��ϵ�����������ӣ�Fe(OH)3��ClO����OH����FeO42����Cl����H2O��
��д������ƽʪ���Ƹ�����ط�Ӧ�����ӷ���ʽ��____________________________
________________________________________________________________________��
��ÿ����1 mol FeO42��ת��________mol���ӣ�����Ӧ������ת����0.3 mol���ӣ���ԭ��������ʵ���Ϊ________mol��
�۵����£��ڸ���������Һ�м���KOH�����Ϳ������������(K2FeO4)��˵��ʲô����_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������������������ˮ��ҵ��ˮ����������������������Ͽ졣ʵ���ҿ��ö�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�
(1)�ڢٲ��в��������������ô�������ԭ����(�û�ѧ����ʽ��ʾ)________________________________________��
(2)KOH��KClO3��MnO2���۷�Ӧ����ī��ɫK2MnO4�Ļ�ѧ����ʽΪ________________________________________��
(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ������MnO4����MnO2����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ________(��ȷ��0.1%)��
(4)�ڢݲ����ȹ��˵�Ŀ����______________________________________��
(5)�ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ��________��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���Ϊ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о�̼���仯������ۺ����öԴٽ���̼���Ĺ���������Ҫ�����塣���������֪ʶ�о�̼���仯��������ʡ�
��1�����������ҹ���������̼���о�ȡ���ش��չ���õ绡���ϳɵ�̼�����г����д���̼�����������ʣ�������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ��______C��________K2Cr2O7��________����________CO2����________K2SO4��________Cr2��SO4��3��________H2O��
����ɲ���ƽ������ѧ����ʽ��
����������ѧ����ʽ�ϱ���÷�Ӧ����ת�Ƶķ�������Ŀ��
��2������ʱ����CO��ԭMgSO4���Ʊ��ߴ�MgO��
��750��ʱ����������к������ʵ���SO2��SO3����ʱ��Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
����MgO���Ƴɡ�þ���������Ρ�ȼ�ϵ�أ���װ��ʾ��ͼ��ͼ��a����ʾ���õ�ط�Ӧ�����ӷ���ʽΪ________________________________________��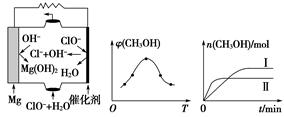
��a���������� ����b��������������c��
��3��������̼�ϳɼ״���̼���ŵ��·���CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪCO2��g����3H2��g��  CH3OH��g����H2O��g������H��
CH3OH��g����H2O��g������H��
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK��________��
��ȡ��ݵ����CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״�����������գ�CH3OH���뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��b����ʾ��������CO2ת��Ϊ�״���Ӧ�Ħ�H________�������������������0��
�������ֲ�ͬ�����·�����Ӧ�����CH3OH�����ʵ�����ʱ��仯��ͼ��c����ʾ�����ߢ��Ӧ��ƽ�ⳣ����С��ϵΪK��________K�����������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
H2O2�ڹ�ҵ��ũҵ��ҽҩ�϶��й㷺����;��
��1��H2O2�Ƕ�Ԫ���ᣬд����һ���ĵ��뷽��ʽ ���ڶ����ĵ���ƽ�ⳣ������ʽKa2�� ��
��2���������ʶ�������H2O2�ֽ�Ĵ�����һ�ֹ۵���Ϊ���ڷ�Ӧ�����д����ȱ�H2O2��������ԭ�������ֱ�H2O2��ԭ�������������������ʶ�����H2O2�ֽ�Ĵ������ڷ�Ӧ�������ȱ���������ԭ���� ��
��I�� ��Fe3�� ��Cu2�� ��Fe2��
��3���ü�������ȼ�ϵ�غϳ�H2O2������Ч�ʸߣ�����Ⱦ���ص㡣����ܷ�ӦΪ��
H2 + O2 + OH�� �� H2O + HO2����д��������Ӧʽ�� ��
��4��H2O2��һ�ֻ����Ѻõ�ǿ����������Ʒ�ˮ����Ҫ��Cu2����Ni2������������Fe3����Fe2����Cr3�� �ȣ��Ʊ���������һ���������£�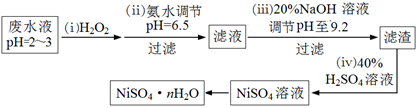
�ٵڣ�������������H2O2��Ӧ�����ӷ���ʽ ��
�ڵڣ��������������е���Ҫ�ɷ���ҽ���ϵ���;�� ��
��Ϊ�ⶨNiSO4��n H2O����ɣ���������ʵ�飺��ȡ2.627g��Ʒ�����Ƴ�250.00 mL��Һ��ȷ��ȡ���Ƶ���Һ25.00 mL����0.04000 mol��L��1��EDTA��Na2H2Y������Һ�ζ�Ni2+�����ӷ���ʽΪNi2��+ H2Y2����NiY2��+ 2H����������EDTA����Һ25.00 mL��������������Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӡ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ���⡣
��1���õ绡���ϳɵĴ�������̼�ܳ����д�����̼�����������ʣ������ֿ������������������ᴿ������ɸ÷�Ӧ�Ļ�ѧ����ʽ��(����ƽ���ϵ�����ں�����)
__ C+ __ KMnO4+ ___ H2SO4��___CO2��+ ___MnSO4 + ___K2SO4+ ___H2O
����Ӧ����2��408��1024�����ӷ���ת��ʱ����ԭ��������Ϊ
��2������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2 L�ĺ����ܱ������У����з�ӦCO��g����H2O��g�� CO2��g����H2��g�����õ������������ݣ�
CO2��g����H2��g�����õ������������ݣ�
| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | x | 2��0 | 1��6 | 2��4 | 6 |
| 2 | 900 | 2��0 | 1��0 | 0��4 | 1��6 | 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һδ��ƽ��������ԭ��Ӧ��
KClO3��PH3��H2SO4��K2SO4��H3PO4��H2O��X
��1���÷�Ӧ�Ļ�ԭ����_________��
��2����֪0.2 mol KClO3�ڷ�Ӧ�еõ�1 mol��������X����X�Ļ�ѧʽ��__________��
��3������������Ӧ����֪__________________����д��ţ���
| A�������ԣ�KClO3��H3PO4 | B�������ԣ�H3PO4��KClO3 |
| C����ԭ�ԣ�PH3��X | D����ԭ�ԣ�X��PH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������Ҫ������������ԭ��������ˮ��Һ�ֳ�Ϊ˫��ˮ��������������ɱ����Ư�ȡ�ij��ѧ��ȤС��ȡһ�����Ĺ���������Һ��ȷ�ⶨ�˹�������ĺ�������̽���˹�����������ʡ�

��.�ⶨ��������ĺ���
��1��ȡ10.00 mL�ܶ�Ϊ�� g/mL�Ĺ���������Һϡ����250mL����ȡϡ�ͺ�Ĺ���������Һ25.00mL����ƿ�У�����ϡ�����ữ��������ˮϡ�ͣ��������������ø�����ر���Һ�ζ�����������д���ζ������з�����Ӧ�����ӷ���ʽ�� _____________________________________________________
��2���ظ��ζ����Σ�ƽ������c mol/L KMnO4����ҺV mL����ԭ����������Һ�й����������������Ϊ____________��
��.̽���������������
��1��H2O2��ͭ���й�̽��ʵ�飺��ͭ˿�����������ữ��H2O2��Һ�У�ͭ��Ѹ����������Һ������ͬʱ�����������壬������������ʹ���ľ����ȼ����Ӧ�����ӷ���ʽΪ��_________________________��
�ڽ�ͭ˿����H2O2��Һ�У�û�����ݲ�����������Һʱ���۲쵽ͭ˿��������������壬��������ʹ���ľ����ȼ���ڸñ仯��ͭ˿�����������__________��д������������һ����ķ�Ӧ�Ļ�ѧ����ʽ__________________________��
��2���������õ���ͨ���Ũ��NaOH��H2O2�Ļ��Һ�У��ڵ��ܿ�����Һ�ĽӴ�������˸�ĺ����֣�������Ϊͨ������Һ�в�����ClO����H2O2��ԭ���������ҷ�Ӧ,���������ϸߵ������ӣ�������ת��Ϊ��ͨ�����ӣ�������������Ժ��ų���ClO����H2O2��Ӧ�����ӷ���ʽ��___________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com