
ЎѕМвДїЎїТСЦЄўЩЎ«ўвєЕФЄЛШФЪЦЬЖЪ±нЦРµДО»ЦГИзНјЎЈКФ»ШґрПВБРОКМвЈє

(1)ЙПКцФЄЛШЦРКфУЪdЗшµДУРЈє________(Мо±аєЕ)ЎЈ
(2)ўЪЎўўЫЎўўЬИэЦЦФЄЛШµДµЪТ»µзАлДЬУЙґуµЅРЎµДЛіРтОЄ________(УГФЄЛШ·ыєЕ±нКѕ)ЎЈ
(3)ЗлёщѕЭµИµзЧУМеФАнЈ¬РґіцУЙўЪЎўўЫБЅЦЦФЄЛШЧйіЙµДґшУРТ»ёцµҐО»ёєµзєЙАлЧУµДµзЧУКЅЈє_____ЎЈ
(4)ўаєЕФЄЛШФЪФЄЛШЦЬЖЪ±нЦРµДО»ЦГКЗ___Ј¬ўвєЕФЧУґ¦УЪ»щМ¬К±єЛНвµзЧУЕЕІјКЅОЄ____Ј¬ТСЦЄФЄЛШўвєНўЯµДµзёєРФ·Ц±рОЄ1.9єН2.5Ј¬ФтўвУлўЯРОіЙµД»ЇєПОпКфУЪ________(МоЎ°АлЧУЎ±»тЎ°№ІјЫЎ±)»ЇєПОпЎЈ
(5)ўЫєЕФЄЛШФЧУУлўЩєЕФЄЛШФЧУРОіЙµДФЧУёцКэ±ИОЄ1ЎГ3µД·ЦЧУXµДїХјд№№РНОЄ________Ј¬XФЪўЩУлўЬРОіЙµД»ЇєПОпYЦРµДИЬЅв¶ИєЬґуЈ¬ЖдЦчТЄФТтКЗ___________Ј¬X·ЦЧУЦРЦРРДФЧУОЄ________ФУ»ЇЈ¬X·ЦЧУУлўвєЕФЄЛШ¶ФУ¦µД¶юјЫСфАлЧУРОіЙµДЕдАлЧУµД»ЇС§КЅОЄ______________ЎЈ
(6)ўЭєЕФЄЛШФЧУУлTlФЄЛШРОіЙµДѕ§МеµДѕ§°ыИзНјЛщКѕЈ¬ёГОпЦКµД»ЇС§КЅОЄ______________Ј¬ИфєцВФTlФЧУЈ¬ФтґЛѕ§МеЦРўЭµДїХјдЅб№№ёъДДЦЦіЈјыОпЦКµДѕ§МеЅб№№Т»СщЈї______________ЎЈТСЦЄёГѕ§МеµДГЬ¶ИОЄ¦С gЎ¤cmЈ3Ј¬°ў·ьјУµВВЮіЈКэОЄNAЈ¬ФтёГѕ§МеЦРѕ§°ы±Яі¤ОЄ________ pm(Ц»РґјЖЛгКЅ)ЎЈ

Ўѕґр°ёЎїўаўб )N>O>C [ЎГCNЎГ]Ј µЪ4ЦЬЖЪµЪўшЧе 1s22s22p63s23p63d104s1 №ІјЫ ИэЅЗЧ¶РО Л®єН°±Жш·ЦЧУјдДЬРОіЙЗвјьЎўЛ®єН°±Жш·ЦЧУ¶јКЗј«РФ·ЦЧУ sp3 [Cu(NH3)4]2Ј« NaTl ЅрёХКЇ ![]()
ЎѕЅвОцЎї
ЈЁ1Ј©dЗш°ьАЁўуBЈўчBЈ¬ТФј°ўшЧеФЄЛШЈ¬ёщѕЭМвЦРЛщёшЦЬЖЪ±нЈ¬О»УЪdЗшµДФЄЛШКЗўаўбЈ»
ЈЁ2Ј©ўЪОЄCЈ¬ўЫОЄNЈ¬ўЬОЄOЈ¬Н¬ЦЬЖЪґУЧуПтУТµЪТ»µзАлДЬЦрЅҐФцґуЈ¬µ«ўтA>ўуAЈ¬ўхA>ўцAЈ¬ТтґЛЛіРтКЗN>O>CЈ»
ЈЁ3Ј©ёГОўБЈКЗCNЈЈ¬CєНNЦ®јд№ІУГИэёцµзЧУ¶ФЈ¬ЖдµзЧУКЅОЄ![]() Ј»
Ј»
ЈЁ4Ј©ўаєЕФЄЛШОЄFeЈ¬О»УЪµЪЛДЦЬЖЪўшЧеЈ»ўвєЕФЄЛШОЄCuЈ¬»щМ¬єЛНвµзЧУЕЕІјКЅОЄ1s22s22p63s23p63d104s1Ј»ўЯєЕФЄЛШОЄSЈ¬БЅЦЦФЄЛШµзёєРФІоЦµОЄ(2.5Ј1.9)=0.6<1.7Ј¬БЅФЧУјд№№іЙ№ІјЫјьЈ¬јґёГ»ЇєПОпОЄ№ІјЫ»ЇєПОпЈ»
ЈЁ5Ј©ўЫєЕФЄЛШОЄNЈ¬ўЩєЕФЄЛШОЄHЈ¬ФЧУёцКэ±ИОЄ1Јє3Ј¬јґёГ»ЇєПОпОЄNH3Ј¬їХјд№№РНОЄИэЅЗЧ¶РОЈ»ўЩўЬРОіЙµД»ЇєПОпОЄH2OЈ¬NH3ФЪЛ®ЦРИЬЅв¶ИґуµДФТтКЗNH3єНH2O¶јКЗј«РФ·ЦЧУЈ¬NH3УлH2OРОіЙ·ЦЧУјдЗвјьЈ¬ФцјУNH3µДИЬЅв¶ИЈ»NH3ЦРЦРРДФЧУNУР3ёц¦ТјьЈ¬№ВµзЧУ¶ФКэОЄ(5Ј3ЎБ1)/2=1Ј¬јЫІгµзЧУ¶ФКэОЄ4Ј¬ФУ»ЇАаРНОЄsp3Ј»NH3УлCu2Ј«РОіЙЕдАлЧУЈ¬Cu2Ј«ЦРРДАлЧУЈ¬ЕдО»КэОЄ4Ј¬Жд»ЇС§КЅОЄ[Cu(NH3)4]2Ј«Ј»
ЈЁ6Ј©ўЭєЕФЄЛШОЄNaЈ¬ёщѕЭѕ§°ыЅб№№Ј¬NaО»УЪѕ§°ыµДАвЙПЎўѕ§МеДЪІїЈ¬ёцКэОЄ12ЎБ1/4Ј«5=8Ј¬TiО»УЪѕ§°ыµД¶ҐµгЎўГжРДєНДЪІїЈ¬ёцКэОЄ8ЎБ18Ј«6ЎБ1/2Ј«4=8Ј¬»ЇС§КЅОЄNaTiЈ»єцВФTiФЧУЈ¬їХјд№№РНУлЅрёХКЇµДЅб№№Т»СщЈ»ѕ§°ыµДЦКБїОЄ![]() Ј¬ѕ§°ыµДМе»эОЄ(aЎБ10Ј10)cm3Ј¬ёщѕЭГЬ¶ИµД¶ЁТеѕ§°ыµД±Яі¤ОЄ
Ј¬ѕ§°ыµДМе»эОЄ(aЎБ10Ј10)cm3Ј¬ёщѕЭГЬ¶ИµД¶ЁТеѕ§°ыµД±Яі¤ОЄ![]() pmЎЈ
pmЎЈ



| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїПВБРАлЧУјмСйµД·Ѕ·ЁХэИ·µДКЗ
A. ДіИЬТє![]() УР°ЧЙ«іБµнЈ¬ЛµГчФИЬТєЦРУРClЈ
УР°ЧЙ«іБµнЈ¬ЛµГчФИЬТєЦРУРClЈ
B. ДіИЬТє![]() УР°ЧЙ«іБµнЈ¬ЛµГчФИЬТєЦРУРSO42Ј
УР°ЧЙ«іБµнЈ¬ЛµГчФИЬТєЦРУРSO42Ј
C. ДіИЬТє![]() УРА¶Й«іБµнЈ¬ЛµГчФИЬТєЦРУРCu2+
УРА¶Й«іБµнЈ¬ЛµГчФИЬТєЦРУРCu2+
D. ДіИЬТє![]() ЙъіЙОЮЙ«ЖшМеЈ¬ЛµГчФИЬТєЦРУРCO32Ј
ЙъіЙОЮЙ«ЖшМеЈ¬ЛµГчФИЬТєЦРУРCO32Ј
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїТСЦЄМюXФЪ±к›rПВµДГЬ¶И1.25g/LЈ¬ТєМ¬МюAД¦¶ыЦКБїОЄ106g/molЈ¬CКЗУНЦ¬Фн»Ї·ґУ¦єуµДІъОпЦ®Т»Ј¬ѕЯОьЛ®±ЈКЄ№¦ДЬЈ¬DКЗУР·јПгЖшО¶µДхҐЎЈЛьГЗЦ®јдµДЧЄ»ЇИзПВНјЛщКѕЈЁє¬УРПаН¬№ЩДЬНЕµДУР»ъОпНЁіЈѕЯУРПаЛЖµД»ЇС§РФЦКЈ©
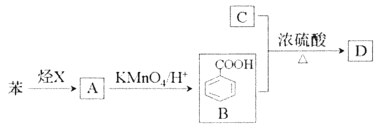
Зл»Шґр
(1)МюXЛщє¬№ЩДЬНЕµДГыіЖКЗ_______ЎЈ
(2)AЎъBµД·ґУ¦АаРНКЗ_________ЎЈ
(3)BУлC°ґ3Јє1·ґУ¦ЙъіЙDµД»ЇС§·ЅіМКЅОЄ_______ЎЈ
(4)ПВБРЛµ·ЁХэИ·µДКЗ_______ЎЈ
A.БЪ¶юде±ЅЦ»УРТ»ЦЦЅб№№Ј¬Ц¤Гч±Ѕ»·Ѕб№№ЦРІ»ґжФЪµҐЛ«јьЅ»МжЅб№№
B.ЙПКцБщЦЦУР»ъОп¶јДЬУлH2·ўЙъјУіЙ·ґУ¦
C.їЙУГРВЦЖCu(OH)2јш±рBЎўCЎўD
D.ПаН¬ЦКБїµД±ЅУл±ЅјЧЛбід·ЦИјЙХПыєДµИБїµДO2
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї¶ЎНйµДТ»ВИґъОпУРЛДЦЦЈ¬ФтОмЛбµДЅб№№УР( )ЦЦ
A. 2B. 3C. 4D. 5
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїДіРЈ»ЇС§РЛИ¤РЎЧйОЄСРѕїВИЖшµДРФЦКІўДЈД⹤ҵЦЖ±ёЖЇ°Ч·ЫЈ¬ЙијЖБЛПВБРЧ°ЦГЅшРРКµСйЎЈТСЦЄЈєўЩAЦР·ґУ¦ОЄ KClO3Ј«6HCl(ЕЁ)=KClЈ«3Cl2ЎьЈ«3H2OЈ»
ўЪКЇ»ТИйµДЦчТЄіЙ·ЦОЄCa(OH)2Ј¬ЖдЛыФУЦКІ»ІОУл·ґУ¦ЎЈ

ЈЁ1Ј©BЧ°ЦГЧчУГ____ЎЈКµСйЅбКшєуЈ¬БўјґЅ« B ЦРИЬТєµОјёµОФЪЧПЙ«КЇИпКФЦЅЙПЈ¬їЙ№ЫІмµЅµДПЦПуКЗ_____ЎЈ
ЈЁ2Ј©Ч°ЦГCµДКµСйДїµДКЗСйЦ¤ВИЖшКЗ·сѕЯУРЖЇ°ЧРФЈ¬ОЄґЛCЦРIЎўIIЎўIIIґ¦ТАґО·ЕИлµДОпЦКХэИ·µДКЗ___ЈЁМо±аєЕЈ©ЎЈ
±аєЕ | I | II | III |
A | ёЙФпµДУРЙ«ІјМх | јоКЇ»Т | КЄИуµДУРЙ«ІјМх |
B | ёЙФпµДУРЙ«ІјМх | ЕЁБтЛб | КЄИуµДУРЙ«ІјМх |
C | КЄИуµДУРЙ«ІјМх | ЕЁБтЛб | ёЙФпµДУРЙ«ІјМх |
D | КЄИуµДУРЙ«ІјМх | јоКЇ»Т | ёЙФпµДУРЙ«ІјМх |
ЈЁ3Ј©ґэEЦРОпЦКНкИ«·ґУ¦єуЈ¬ѕ№эТ»ПµБРјУ№¤ґ¦АнЈ¬µГµЅЖЇ°Ч·ЫСщЖ·Ј¬ЖдЦчТЄіЙ·ЭОЄ___ЈЁМо»ЇС§КЅЈ©
ЈЁ4Ј©FЧ°ЦГµДЧчУГКЗЈЁУГ»ЇС§·ЅіМКЅ±нКѕЈ©____ЎЈ
ЈЁ5Ј©ОЄІв¶ЁЈЁ3Ј©ЦРЛщµГЖЇ°Ч·ЫµДУРР§іЙ·Эє¬БїЎЈіЖИЎagЖЇ°Ч·ЫСщЖ·ИЬЅвЈ¬НщЛщµГИЬТєЦРНЁИл CO2ЦБІъЙъіБµнЧоґуЦµК±Ј¬ёГ№эіМµД»ЇС§·ЅіМКЅОЄ_____Ј¬Иф·ґУ¦ЙъіЙіБµнµДОпЦКµДБїОЄbmolЈ¬ФтёГЖЇ°Ч·ЫЦРУРР§іЙ·ЭµДЦКБї·ЦКэОЄ_____ЈЁУГє¬aЎўbµДКЅЧУ±нКѕЈ©ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїТСЦЄТТЛбУлТТЛбТТхҐµД»мєПОпЦРє¬ЗвµДЦКБї·ЦКэОЄ7%, ЖдЦРє¬OБїКЗ
A. 42%B. 51%C. 48.6%D. 91.9%
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїТСЦЄAЎўBЎўCЎўDЛДЦЦ¶МЦЬЖЪФЄЛШФЪЦЬЖЪ±нЦРµДПа¶ФО»ЦГИзУТ±н,ЖдЦРDµДФЧУРтКэКЗAµДФЧУРтКэµД3±¶ЎЈAУлDЧйіЙµДОпЦККЗТ»ЦЦі¬УІДНДҐНїІгІДБП,Нј3ОЄЖдѕ§МеЅб№№ЦРЧоРЎµДЦШёґЅб№№µҐФЄЈ¬ЖдЦРµДГїёцФЧУЧоНвІгѕщВъЧг8µзЧУОИ¶ЁЅб№№ЎЈПВБРУР№ШЛµ·ЁХэИ·µДКЗ

A. AУлDЧйіЙµДОпЦКµД»ЇС§КЅОЄBP,КфУЪАлЧУѕ§Ме
B. AУлDЧйіЙµДОпЦКИЫµгёЯЈ¬ЗТИЫИЪЧґМ¬ПВДЬµјµз
C. AУлDЧйіЙµДОпЦКЦРФЧУјдТФ№ІјЫјьБ¬ЅУЈ¬КфУЪФЧУѕ§Ме
D. AУлDЧйіЙµД»ЇєПОпѕ§МеЦРЈ¬ГїёцAФЧУЦЬО§Б¬ЅУ1ёцDФЧУ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїУЙ![]() єН
єН![]() ЧйіЙµД»мєПЖшМеУлН¬ОВН¬С№ПВїХЖшµДГЬ¶ИПаµИ
ЧйіЙµД»мєПЖшМеУлН¬ОВН¬С№ПВїХЖшµДГЬ¶ИПаµИ![]() їХЖшµДЖЅѕщПа¶Ф·ЦЧУЦКБїОЄ29,
їХЖшµДЖЅѕщПа¶Ф·ЦЧУЦКБїОЄ29,![]() ФтПВБР№ШПµХэИ·µДКЗ()
ФтПВБР№ШПµХэИ·µДКЗ()
A.»мєПЖшМеЦР,![]() ХјУРµДМе»эґуУЪ
ХјУРµДМе»эґуУЪ![]() ХјУРµДМе»э
ХјУРµДМе»э
B.»мєПЖшМеЦР,![]() Ул
Ул![]() ·ЦЧУёцКэ±ИОЄ1Јє2
·ЦЧУёцКэ±ИОЄ1Јє2
C.»мєПЖшМеЦР,![]() Ул
Ул![]() ЦКБї±ИОЄ15Јє14
ЦКБї±ИОЄ15Јє14
D.»мєПЖшМеЦР,![]() Ул
Ул![]() ГЬ¶И±ИОЄ14Јє15
ГЬ¶И±ИОЄ14Јє15
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїµюµЄ»ЇДЖ(NaN3)іЈУГЧчЖыіµ°ІИ«ЖшДТЦРµДТ©јБЎЈКµСйКТЦЖИЎµюµЄ»ЇДЖµДФАнЎўКµСйЧ°ЦГј°КµСйІЅЦиИзПВЈє
ўЩґтїЄЧ°ЦГDµј№ЬЙПµДРэИыЈ¬јУИИЦЖИЎ°±ЖшЎЈ
ўЪФЩјУИИЧ°ЦГAЦРµДЅрКфДЖЈ¬К№ЖдИЫ»ЇІўід·Ц·ґУ¦єуЈ¬ФЩНЈЦ№јУИИЧ°ЦГDІў№Ш±ХРэИыЎЈ
ўЫПтЧ°ЦГAЦРbИЭЖчДЪідИлјУИИЅйЦКІўјУИИµЅ210Ў«220ЎжЈ¬И»єуНЁИлN2OЎЈ
ўЬАдИґЈ¬ПтІъОпЦРјУИлТТґј(ЅµµНNaN3µДИЬЅв¶И)Ј¬јхС№ЕЁЛхЅбѕ§єуЈ¬ФЩ№эВЛЈ¬ІўУГТТГСПґµУЈ¬БАёЙЎЈ

ТСЦЄЈєIЈ®NaN3КЗТЧИЬУЪЛ®µД°ЧЙ«ѕ§МеЈ¬ОўИЬУЪТТґјЈ¬І»ИЬУЪТТГСЈ»
IIЈ®NaNH2ИЫµг210ЎжЈ¬·Рµг400ЎжЈ¬ФЪЛ®ИЬТєЦРТЧЛ®ЅвЎЈ
Зл»ШґрПВБРОКМвЈє
(1)Ч°ЦГBЦРКў·ЕµДТ©Ж·ОЄ_____________Ј»Ч°ЦГCµДЦчТЄЧчУГКЗ______________________ЎЈ
(2)ІЅЦиўЩЦРПИјУИИНЁ°±ЖшµДДїµДКЗ_____________________________________Ј»ІЅЦиўЪ°±ЖшУлИЫ»ЇµДДЖ·ґУ¦ЙъіЙNaNH2µД»ЇС§·ЅіМКЅОЄ_______________________________ЎЈІЅЦиўЫЦРЧоККТЛµДјУИИ·ЅКЅОЄ ___________(МоЎ°Л®ФЎјУИИЎ±Ј¬Ў°УНФЎјУИИЎ±)ЎЈ
(3)N2OїЙУЙNH4NO3ФЪ240Ў«245Ўж·ЦЅвЦЖµГ(ПхЛб淋ДИЫµгОЄ169.6Ўж)Ј¬ФтїЙСЎФсµДЖшМе·ўЙъЧ°ЦГКЗ(МоРтєЕ)___________ЎЈ

(4)ЙъіЙNaN3µД»ЇС§·ЅіМКЅОЄ _____________________________________ЎЈ
(5)НјЦРТЗЖчaУГµДКЗМъЦК¶шІ»УГІЈБ§Ј¬ЖдЦчТЄФТтКЗ__________________ЎЈ
(6)ІЅЦиўЬЦРУГТТГСПґµУµДЦчТЄДїµДКЗ_______________________________ЎЈ
(7)КµСйКТУГµО¶Ё·ЁІв¶ЁµюµЄ»ЇДЖСщЖ·ЦРNaN3µДЦКБї·ЦКэЈєўЩЅ«2.500 gКФСщЕдіЙ500.00 mLИЬТєЎЈўЪИЎ50.00 mLИЬТєЦГУЪЧ¶РОЖїЦРЈ¬јУИл50.00 mL 0.1010 molЎ¤L-1(NH4)2Ce(NO3)6ИЬТєЎЈўЫід·Ц·ґУ¦єуЈ¬Ѕ«ИЬТєЙФПЎКНЈ¬ПтИЬТєЦРјУИл8 mLЕЁБтЛбЈ¬µОИл3µОБЪ·Ж†ЄЯшЦёКѕТєЈ¬УГ0.0500molЎ¤L-1(NH4)2Fe(SO4)2±кЧјИЬТєµО¶Ё№эБїµДCe4+Ј¬ПыєДИЬТєМе»эОЄ29.00mLЎЈІв¶Ё№эіМµД·ґУ¦·ЅіМКЅОЄЈє2(NH4)2Ce(NO3)6+2NaN3=4NH4NO3+2Ce(NO3)3+2NaNO3+3N2ЎьЈ»Ce4++Fe2+=Ce3++Fe3+Ј»КФСщЦРNaN3µДЦКБї·ЦКэОЄ_______________ЎЈ
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com