
��19�֣���֪A��B��C��D��E��F��C��H����Ԫ��λ��Ԫ�����ڱ�ǰ�ĸ����ڡ�A��B��C�����ַǽ���Ԫ�أ�A��B��ԭ�ӵĺ��������֮����Cԭ�ӵĺ�������������A��B��C���γ����ӻ����B��Hλ��ͬһ���壬D�ĵ�������ѧ��ѧ���������Խ�����E�ĵ������ճ���������;��㷺�Ľ�������۵�������G��������Fԭ�ӵ�������Ӳ��p��������s��������������D��H��Fλ��ͬһ������ԭ��������������A��C��E��G��H�Ļ�̬ԭ����δ�ɶԵ���������������������ͬ�����û�ѧ����ش��������⣺
��1��A��B��C�γɵĴ˻������д��ڵĻ�ѧ���������� ��E�����ڳ���������A��B��C�γɵ���һ�������Ũ��Һ�����ۻ���������E�����ڴ˻������ϡ��Һ�з�����Ӧ�����ӷ���ʽΪ ��E�Ļ�̬�����Ų�ʽΪ ��E3+��E2+���ȶ��Դ�СΪ ��
��2��B��C��D��H����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ ���縺����С�����˳��Ϊ ��
��3��A��C��G���γ�һ����Է�������Ϊ46��һԪ������ӣ�������д��ڵ�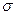 ����
���� ������Ŀ֮��Ϊ ��F��G��Ӧ���⻯���м��ܴ�СΪF��H�� G��H����
������Ŀ֮��Ϊ ��F��G��Ӧ���⻯���м��ܴ�СΪF��H�� G��H����
��4����Ũ�ȡ�����������������������Һ�ֱ���������D�ĵ��ʷ�Ӧ�ų��������ڳ��³�ѹ�µ������Ϊ ����������Һ��ϸպ���DԪ�ص��������ʼ䷢����Ӧ�����ӷ���ʽΪ ��
��5����A��C��F���γ����������������˷���ʽ˵�����ǵ�����ǿ�� ����A��C��F��������Ԫ�ذ�ԭ�Ӹ�����Ϊl��3��1��1���һ�ֻ������ˮ��Һ�����ԣ������Һ�и������ӵ�Ũ���ɴ�С��˳��Ϊ �������Һ�е�������������������Һʱ������Ӧ�����ӷ���ʽΪ ��
��19�֣�
��1�����Ӽ������ۼ���2�֣�
3Fe+8H++2NO-3=3Fe2-+2NO��+4H2O ��������2��
1s22s22p63p63d44s2[Ar]3d54s2��1�֣�
Fe3+>Fe2-
��2��N>O>P>Al��1�֣���Al<P<N<O ��������1��
��3��4��1 ��������1��
> ��������1��
��4��3��1��1�֣���
Al3-+3AlO-2+6H2O=4Al��OH��3�� ��������2��
��5��2H++SO2-3=SO2��+H2O��H++HSO-4=SO2��+H2O�����������֣�2�֣���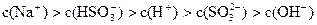
Ba2++2HSO-2=BaSO3��+SO2-3+2H2O ��������2��
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
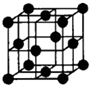
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com