

���� ��1�����ڿ�����Ҳ�ж�����̼��ˮ�֣������B�����þ��Dz������ǽ���ģ�
��2����������Ļӷ��Կ��ǣ�
��3�����ڷ�Ӧ������ƿ�д����ж�����̼������һ�����Ŀ�������Ϊ���������ǵģ�
��4���ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊ�˽�������̼ȫ���Ϲ�ȥ��
��5������U�ܵ��������������������ɵĶ�����̼�����������ݶ�����̼���������̼���Ƶ�����������̼���Ƶ�����������Ʒ�������ɣ�
��� �⣺��1��U���еļ�ʯ����Ϊ�����շ�Ӧ���ɵĶ�����̼����������Ҳ���ڶ�����̼�������B�����þ��Ƿ�ֹ�����еĶ�����̼��ˮ�ֽ���U�ܣ��Խ��������
�ʴ�Ϊ����ֹ�����е�CO2��ˮ������U���У�
��2������������лӷ��ԣ�Ҳ�����Ŷ�����̼����U�ܣ�������Ϊ�Ƕ�����̼�����Զ�����̼������ƫ�������̼���Ƶ�����Ҳ��ƫ��ģ����Խ����ƫ��
�ʴ�Ϊ��ƫ�ߣ�
��3�����ڷ�Ӧ������ƿ�д����ж�����̼������һ�����Ŀ������ǽ������Ķ�����̼��ȫ����U�ܣ���ȱ�ٸò��裬������̼�������٣���Na2CO3�������������������ƫ�ͣ�
�ʴ�Ϊ���ѷ�Ӧ������CO2ȫ������U���У�ƫ�ͣ�
��4���ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬˵��������̼�Ѿ���ȫ���ŵ�U���У�
�ʴ�Ϊ���жϷ�Ӧ������CO2�Ƿ�ȫ���ų�������U���еļ�ʯ�����գ�
��5������Ҫ̼���Ƶ�����ΪX����
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 44
X d-b
���X=$\frac{106��d-b��}{44}$g��
���������д�������������ļ���ʽ=$\frac{106��d-b��}{44a}$��100%��
�ʴ�Ϊ��$\frac{106��d-b��}{44a}$��100%��
���� ���⿼����������ɶ����ⶨ��ʵ�鷽����ƺͷ����жϣ���Ҫ�����Ƽ��仯���������Ӧ�ã����׳����ĵط��Dz���Ƹ����B�����ǿ����еĶ�����̼��ˮ��Ҳ�ܹ�����U���У���ʹ���ƫ����Ŀ�Ѷ��еȣ�


 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.2Ħ/�� | B�� | 0.4Ħ/�� | C�� | 0.8Ħ/�� | D�� | 1.6Ħ/�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
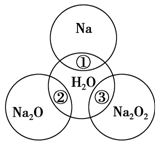 ��ͼ��ʾ����ԲȦ�ཻ�IJ��ֱ�ʾԲȦ�ڵ�����������ķ�Ӧ����֪�Ƽ�������������ʵ�����Ϊ0.1mol��ˮ������Ϊ100g��������Һ���ܼ��ı仯�����������Ŀ��
��ͼ��ʾ����ԲȦ�ཻ�IJ��ֱ�ʾԲȦ�ڵ�����������ķ�Ӧ����֪�Ƽ�������������ʵ�����Ϊ0.1mol��ˮ������Ϊ100g��������Һ���ܼ��ı仯�����������Ŀ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ñ���ȡ��ˮ�е���ʱ������ı���Һ�ӷ�Һ©���¿ڵ��� | |
| B�� | ʹ������ƿ�ĵ�һ���������Ƚ�����ƿ������ˮϴ�Ӻ��� | |
| C�� | ��10 mL��Ͳ��ȡ9.2 mL NaCl��Һ | |
| D�� | ����ij��Һ�Ƿ���SO42-ʱ��Ӧȡ��������Һ�����μ���BaCl2��Һ��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.16mol | B�� | 0.21mol | C�� | 0.30mol | D�� | 0.48mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������� | B�� | ��ԭ���� | C�� | �������� | D�� | ��ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3-C��CH��CH2=CH-CH=CH2 | B�� | CH3-CH=CH2�� 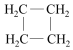 | ||
| C�� | 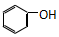 �� �� 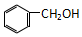 | D�� | 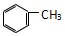 �� ��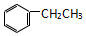 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���ᴿ������ | ���� | �����Լ� | ���ӷ��� |
| A | Fe | Al | NaOH��Һ | ���� |
| B | �������� | �Ҵ� | ˮ | ˮϴ����Һ |
| C | CO2 | SO2 | ����NaHCO3��Һ��ŨH2SO4 | ϴ�� |
| D | NH4Cl��aq�� | Cu2+��aq�� | NaOH��Һ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��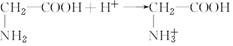 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com