
��1���������廯�ƺͽ�ŨH2SO4�����Ϊԭ�ϣ���ʵ�����Ʊ�1���嶡�飬�����鷴Ӧ�IJ��ָ��������֪��NaCl+H2SO4(Ũ)=NaHSO4+HCl�������������װ�ã����мг�������������������ȴˮ��û�л�������ش��������⣺
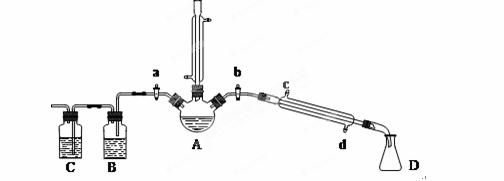
(1)����A�������� ��
(2)�ر�a��b����ͨ��ֱ�����ܵ�����ˮ����A����30���ӣ��Ʊ�1���嶡�顣д���÷�Ӧ�Ļ�ѧ����ʽ ��
(3)�����ϣ�������Ӧ�������ﻹ�����У����ѡ�1����ϩ���廯��ȡ�Ϩ��A���ƾ��ƣ�����ֱ�������Ϸ��������ӣ���a���������ȼ�����Ӧֱ����ȴ��ͨ��B��Cװ�ü��鲿�ָ����B��C��Ӧʢ�ŵ��Լ��ֱ��� �� ��
(4)��ʵ������У�����A��Һ������ɫ��ɺ�ɫ���ú�ɫ������Ũ���ᷴӦ�Ļ�ѧ����ʽΪ ��������ֱ�����ܵ��϶�����һ����װ���ռ���ʯ�ҵĸ���ܣ�������Ⱦ������
(5)����л�����������£�
| ���� | �۵�/0C | �е�/0C |
| 1������ | -89.5 | 117.3 |
| 1���嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1����ϩ | -185.3 | -6.5 |
Ϊ�˽�һ������1���嶡�飬��������������ʵ�飺����ƿ��ȴ��ȥ��ֱ�������ܣ����ϴ��¶ȼƵ���Ƥ�����ر�a����b����ͨ�����ܵ�����ˮ��ʹ��ˮ�� ����c��d�������룬Ѹ�������¶��� �棬�ռ�������֡�
(6)��ʵ������ȡ1��������NaBr�ֱ�Ϊ7.4 g��13.0 g�������Ĵֲ��ᆳϴ�ӡ�������ٴ�����õ�9.6 g 1���嶡�飬��1���嶡��IJ����� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������䡱���ҹ��������ص㹤�̣����������ָ��Ȼ��������Ҫ�ɷ��Ǽ��顣��ҵ�Ͻ�̼��ˮ�ڸ����·�Ӧ�Ƶ�ˮú����ˮú������Ҫ�ɷ���CO��H2�����ߵ������Ϊ11����֪1 mol CO������ȫȼ������CO2����ų�282.6 kJ������1 mol������ȫȼ������Һ̬ˮ�ų�285.8 kJ������1 mol CH4������ȫȼ������CO2�����Һ̬ˮ�ų�889.6 kJ������
(1)д��������ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��
________________________________________________________________________________________________________________________________________________��
(2)��1 mol CH4������ȫȼ������CO2�����ˮ�������ų�������________(����ڡ������ڡ���С�ڡ�)889.6 kJ��
(3)����ˮú���������ɷ֣���ͬ״�������õ���ȵ�����������ˮú�������������ԼΪ(������)________��
(4)�������ݺͼ���˵��������Ȼ�����ˮú����ȼ�ϣ�ͻ�����ŵ���____________________________________________________ ____________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A��ԭ�Ӿ�����ԭ�Ӽ��Թ��ۼ�����
B��ϡ�������γɵľ������ڷ��Ӿ���
C���ɱ�����ʱ�������ڹ��ۼ��ᷢ������
D������Ԫ�غͷǽ���Ԫ���γɵĻ����ﲻһ�������ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ� ��
A�����ᱡ�ɴ�����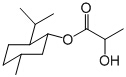 �����ܷ���ˮ�⡢��������ȥ��Ӧ
�����ܷ���ˮ�⡢��������ȥ��Ӧ
B����ȩ�ͱ�ϩȩ��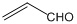 ������ͬϵ�������������ַ�Ӧ��IJ���Ҳ����ͬϵ��
������ͬϵ�������������ַ�Ӧ��IJ���Ҳ����ͬϵ��
C�����ۺ���ά�����������ȫˮ���IJ��ﶼ��������
D��CH3COOCH2CH3��CH3CH2COOCH3��Ϊͬ���칹�壬1H-NMR����ʾ���߾� �����ֲ�ͬ����ԭ����������ԭ�ӵı�����ͬ���ʲ�����1H-NMR������
�����ֲ�ͬ����ԭ����������ԭ�ӵı�����ͬ���ʲ�����1H-NMR������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʵ�����У����г�ȥ���ʵķ�����ȷ����( )
�������л��� ϩ��ͨ��������һ�������·�Ӧ��ʹ��ϩת��Ϊ���飻
ϩ��ͨ��������һ�������·�Ӧ��ʹ��ϩת��Ϊ���飻
�ڳ�ȥ��������������������ñ���̼������Һϴ�ӣ���Һ���������
�۳�ȥCO2��������SO2������ͨ��ʢ����̼������Һ��ϴ��ƿ��
�ܳ�ȥ�Ҵ��������������������ʯ�ң�����
���屽�л����壬����KI��Һ������������ȡ���壻
���������л���ŨHNO3��ŨH2SO4�����䵹��NaOH��Һ�У����ú��ٹ��ˡ�
A���٢ڢۢ� B���ڢ� C���ڢۢ� D���ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ���ܴ����������
A��pH=1����Һ�У�Fe2+��NO3����SO42����Na+
B��c(H+)/c(OH��)=1012����Һ�У�NH4+��Al3+��NO3����Cl��
C����ˮ�����c(H+)=1��10��14mol��L��1����Һ�У�Ca2+��K+��Cl����HCO3��
D��c(Fe3+)=0.1mol��L��1����Һ�У�K+��ClO����SO42����SCN��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼʾ���Ӧ�������������

A����ʾij���ȷ�Ӧ�ֱ����С�����������·�Ӧ�����е������仯
B����ʾ0.1000mol��L-1NaOH��Һ�ζ�20.00mL 0.1000mol��L-1CH3COOH��Һ���õ��ĵζ�����
C����ʾKNO3���ܽ�����ߣ�ͼ��a����ʾ����Һ��80 ��ʱKNO3�IJ�������Һ
D����ʾij���淴Ӧ����������淴Ӧʱ��仯�����ߣ���ͼ֪tʱ��Ӧ��ת�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���з�Ӧ���ʱ�Ϊ��
H2 (g) + I2 (s) = HI (g) ��r H
I2 (s) = HI (g) ��r H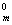 = 25.9 kJ��mol-1
= 25.9 kJ��mol-1
 H2 (g) = H (g) ��r H
H2 (g) = H (g) ��r H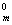 = 218 kJ��mol-1
= 218 kJ��mol-1
 I2 (g) = I (g) ��r H
I2 (g) = I (g) ��r H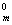 = 75.7 kJ��mol-1
= 75.7 kJ��mol-1
I2 (s) = I2 (g) ��r H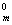 = 62.3 kJ��mol-1
= 62.3 kJ��mol-1
���㷴Ӧ H (g) + I (g) = HI (g) ���ʱ䦤r H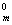 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����� ��һ����Ҫ�Ļ���ԭ�ϣ����й����������˵������ȷ���ǣ�˫ѡ�� (����)
��һ����Ҫ�Ļ���ԭ�ϣ����й����������˵������ȷ���ǣ�˫ѡ�� (����)
A.������DZ���ͬϵ��
B.�������Ը��������Һ�����������
C.�ڹ��յ������£��������Cl2����ȡ ����Ӧ���ɵ��ȴ���������
����Ӧ���ɵ��ȴ���������
D.��һ�������²��������������ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com