
��֪�����ȼ����Ϊ1190kJ/mol�������б�����ȷ���ǣ� ��
A��2C2H6(g)+7O2 (g) = 4CO2 (g)+6H2O(l)����H=��1190kJ/mol
B��C2H6(g)+7/2O2 (g) =2CO2 (g)+3H2O(g)����H=��1190kJ/mol
C��298k��101kPaʱ��30g����������ȫȼ������CO2�����Һ̬ˮ���ų�1190kJ������
D���÷�Ӧ�У���Ӧ�������е�������С�������������е���������
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ��Ӧ���̷������ʱ仯ͬʱ�����������仯������������ʽ���ֳ������з�Ӧ�ȣ����кܶ��֣�ȼ���ȣ��к��ȵȣ�
��ѧ��Ӧ���̷������ʱ仯ͬʱ�����������仯������������ʽ���ֳ������з�Ӧ�ȣ����кܶ��֣�ȼ���ȣ��к��ȵȣ�| 1 |
| 2 |
| 7 |
| 2 |
| 7 |
| 2 |
| ��ѧ�� | P-P | P-O | O=O | P=O |
| ���ܣ�kJ/mol�� | 197 | 360 | 499 | X |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
1.��֪������(HBrO)�Ľṹ��ʽΪHO��Br����ͼ��ϩ����ˮ�и��ɷַ�Ӧ�ȿ�����A��ͬʱ�ֿ�����B��C2H5Br��

�ش��������⣺
(1)��������![]() �Ľṹ�ɼ�дΪ
�Ľṹ�ɼ�дΪ![]() ����E�Ļ�ѧʽ��__________��
����E�Ļ�ѧʽ��__________��
(2)A�Ľṹ��ʽ��__________��I�Ľṹ��ʽ��__________��
(3)д��Gת��ΪH�Ļ�ѧ����ʽ________________________���䷴Ӧ����Ϊ__________��Ӧ��
(4)������A��I�У������ӳɷ�Ӧ��������__________(����ĸ)��
2.(һ)(1)�Ƽ��ձ�
A.Al2H6��HΪ+1�ۣ�AlΪ-3��
B.Al2H6�ڿ�������ȫȼ�գ�����Ϊ��������ˮ
C.����������Al2H6�ľ���Ϊ���Ӿ���
D.������������ܳ�Ϊδ���Ĵ�����Ϻͻ��ȼ��
(��)A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��Dͬ���壻C��Eͬ���壻B��Cͬ���ڣ�Bԭ���������������������������2��A��B��Ԫ�صĺ˵����֮��������ǵ�ԭ������������֮�ͣ�FԪ����ͬ����Ԫ����ԭ�Ӱ뾶��С������Ԫ�ء�A��B��C��D��E��F�γɵĻ�����ס��ҡ����������졢����������±���ʾ��
������ | �� | �� | �� | �� | �� | �� |
��ѧʽ | A | A | B | D | A2E | DF |
�ش��������⣺
(2)�����ﶡ�ĵ���ʽΪ__________��Fԭ�ӵ����������Ų�ʽΪ__________��
(3)��������ķе�ȼ�__________(��ߡ��͡�)��д�����붡��Ӧ�Ļ�ѧ����ʽ��____________________��
(4)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.����ͼ��ʾ����2molA�����1molB�������һ�ݻ��ɱ���ܱ������У�������Ӧ��2A��g��+B(g) ![]() 2C(g).��Ӧ��ʼʱ�ɻ����Ļ�����λ�����ͼ��ʾ������Ӧ�ﵽƽ��ʱ������λ������ͼ��ʾ���ﵽƽ��ʱ��A��ת����Ϊ ���������µķ�Ӧ��ƽ�ⳣ��Ϊ ��
2C(g).��Ӧ��ʼʱ�ɻ����Ļ�����λ�����ͼ��ʾ������Ӧ�ﵽƽ��ʱ������λ������ͼ��ʾ���ﵽƽ��ʱ��A��ת����Ϊ ���������µķ�Ӧ��ƽ�ⳣ��Ϊ ��

��.(1)��֪298Kʱ��1molC2H6����������ȫȼ�����ɶ�����̼��Һ̬ˮ���ų�����1558.3KJ��д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��2�����ø÷�Ӧ�����һ��ȼ�ϵ�أ�������������Һ���������Һ���ö��ʯī���缫���ڵ缫�Ϸֱ���������������д�������ĵ缫��Ӧʽ ��
��3����ʯī���������������������������ͭ��Һ����ʯī���ϵĵ缫��ӦʽΪ �������ʼʱʢ��1000mL PH=5������ͭ��Һ��25�棩����������һ��ʱ�����Һ��PH��Ϊ1����Ҫʹ��Һ�ָ�����ʼŨ�ȣ�������Һ����仯����������Һ�м��� �����������ƣ���������ԼΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������е�����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(12��)��ѧ��Ӧ�����з������ʱ仯��ͬʱ���������������ı仯�����������ı仯�������ܵ���ʽ���ֳ�����������Ӧ�ȡ����ڷ�Ӧ�������ͬ����Ӧ�ȿ��Է�Ϊ�����֣���ȼ���Ⱥ��к��ȵȡ�
��1�����Ц�H��ʾ����ȼ���ȵ��� ����ʾ�����к��ȵ��� ��
(���H1��������H2���͡���H3����)
A��2H2(g)+O2(g) = 2H2O(l) ����H1
B��C(s)+1/2O2(g) = CO(g)����H2
C��CH4(g)+2O2(g) = CO2(g)+2H2O(g)����H3
D��C(s)+O2(g)= CO2(g) ����H4
E��C6H12O6(s)+6O2(g) = 6CO2(g)+6H2O(l)����H5
F��NaOH(aq)+HCl(aq) = NaCl(aq)+H2O(l)����H6
G��2NaOH(aq)+H2SO4(aq) = Na2SO4(aq)+2H2O(l) ��H7
��2����֪��101kPa��273Kʱ��15g����ȼ������CO2��Һ̬ˮ���ų�akJ�������������Ȼ�ѧ����ʽ��ȷ���� ��
A��C2H6(g)+7/2O2(g) = 2CO2(g)+3H2O(l) ��H= +2akJ/mol
B��C2H6(g)+7/2O2(g) = 2CO2(g)+3H2O(g) ��H= -2akJ/mol
C��2C2H6(g)+7O2(g) = 4CO2(g)+6H2O(l) ��H= -4akJ/mol
D��2C2H6(g)+7O2(g) = 4CO2(g)+6H2O(g) ��H= -4akJ/mol
��3����һ���о���������ѧ��Ӧ�������仯(��H)�뷴Ӧ���������ļ����йأ����ܿ��Լ�����Ϊ�Ͽ�1mol��ѧ��ʱ�������յ����������±��Dz��ֻ�ѧ���ļ������ݣ�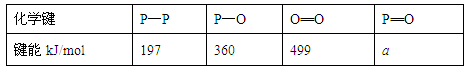
��֪����(P4)��ȼ����Ϊ2378.0kJ/mol��������ȫȼ�յIJ���(P4O10)�Ľṹ����ͼ��ʾ�����ϱ���a= ��
��4����25�桢101kPa�£�1g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
(12��)��ѧ��Ӧ�����з������ʱ仯��ͬʱ���������������ı仯�����������ı仯�������ܵ���ʽ���ֳ�����������Ӧ�ȡ����ڷ�Ӧ�������ͬ����Ӧ�ȿ��Է�Ϊ�����֣���ȼ���Ⱥ��к��ȵȡ�
��1�����Ц�H��ʾ����ȼ���ȵ��� ����ʾ�����к��ȵ��� ��
(���H1��������H2���͡���H3����)
A��2H2(g)+O2(g) = 2H2O(l) ����H1
B��C(s)+1/2O2(g) = CO(g)����H2
C��CH4(g)+2O2(g) = CO2(g)+2H2O(g)����H3
D��C(s)+O2(g)= CO2(g) ����H4
E��C6H12O6(s)+6O2(g) = 6CO2(g)+6H2O(l)����H5
F��NaOH(aq)+HCl(aq) = NaCl(aq)+H2O(l)����H6
G��2NaOH(aq)+H2SO4(aq) = Na2SO4(aq)+2H2O(l) ��H7
��2����֪��101kPa��273Kʱ��15g����ȼ������CO2��Һ̬ˮ���ų�akJ�������������Ȼ�ѧ����ʽ��ȷ���� ��
A��C2H6(g)+7/2O2(g) = 2CO2(g)+3H2O(l) ��H= +2akJ/mol
B��C2H6(g)+7/2O2(g) = 2CO2(g)+3H2O(g) ��H= -2akJ/mol
C��2C2H6(g)+7O2(g) = 4CO2(g)+6H2O(l) ��H= -4akJ/mol
D��2C2H6(g)+7O2(g) = 4CO2(g)+6H2O(g) ��H= -4akJ/mol
��3����һ���о���������ѧ��Ӧ�������仯(��H)�뷴Ӧ���������ļ����йأ����ܿ��Լ�����Ϊ�Ͽ�1mol��ѧ��ʱ�������յ����������±��Dz��ֻ�ѧ���ļ������ݣ�

��֪����(P4)��ȼ����Ϊ2378.0kJ/mol��������ȫȼ�յIJ���(P4O10)�Ľṹ����ͼ��ʾ�����ϱ���a= ��

��4����25�桢101kPa�£�1g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com