
| �� | �� | �� | �� | |
| pH | 12 | 12 | 2 | 2 |
| ��Һ | ��ˮ | ����������Һ | ���� | ���� |
| A�� | �ڢ١�������Һ�зֱ�����Ȼ�茶��壬����Һ��pH������ | |
| B�� | ��������Ģٺ͢�����Һ�ֱ��ˮϡ��100����������Һ��pH���٣��� | |
| C�� | �Ѣ١�������Һ�������Ϻ�������Һ�У�[Cl-]��[NH4+]��[OH-]��[H+] | |
| D�� | ����Һ�ں���Һ�۵������ϣ���Ϻ�������Һ��pH=7 |
���� A��笠��������ư�ˮ�ĵ��룬笠�������NaOH��Ӧһˮ�ϰ���
B����ˮϡ�ʹٽ�һˮ�ϰ��ĵ��룻
C����ˮ��Ũ�ȴ���0.01mol/L��HCl��Ũ��Ϊ0.01mol/L�����ߵ���������Һ�Լ��ԣ�
D�������Ũ�ȴ���0.01mol/L��NaOH��Ũ��Ϊ0.01mol/L�����ߵ���������Һ�����ԣ�
��� �⣺A���ڰ�ˮ�м��Ȼ�茶��壬笠��������ư�ˮ�ĵ��룬��Һ������������Ũ�ȼ�С��pH��С��NaOH��Һ�м��Ȼ�泥�笠�������NaOH��Ӧһˮ�ϰ�����Һ������������Ũ�ȼ�С��pH��С����A����
B����ˮϡ�ʹٽ�һˮ�ϰ��ĵ��룬��Һ������������Ũ�ȱ仯���������Խ�������Ģٺ͢�����Һ�ֱ��ˮϡ��100����������Һ��pH����ǰ�ˮ������Һ��pH���٣��ڣ���B��ȷ��
C����ˮ��Ũ�ȴ���0.01mol/L��HCl��Ũ��Ϊ0.01mol/L�����ߵ������ϣ���ˮ��ʣ�࣬��Һ�Լ��ԣ�������Һ�У�[NH4+]��[Cl-]��[OH-]��[H+]����C����
D�������Ũ�ȴ���0.01mol/L��NaOH��Ũ��Ϊ0.01mol/L�����ߵ������ϣ�������ʣ�࣬���Ի����Һ�����ԣ���pH��7����D����
��ѡB��
���� ���⿼����������ʵĵ��롢�������Һ��Һ�Ķ����жϵȣ���Ŀ�ѶȲ���ע�������������е���ʵ�Ũ���������ӻ�����������Ũ�ȵĹ�ϵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������и߶���9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪�Ҵ���ʯī��������ȼ���ȷֱ�Ϊa��b��c(��Ϊ��ֵ����λ��ΪkJ��mol��1)����Ӧ2C(s��ʯī)��2H2(g)��H2O(l)==C2H5OH(l)���ʱ�Ϊ( )
A��(a��2b��2c) kJ��mol��1
B��(2b��2c��a) kJ��mol��1
C��(b��c��a) kJ��mol��1
D��(a��2b��c) kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=4.3��CH3COOH��CH3COONa�����Һ�У�c��Na+����c��CH3COO-�� | |
| B�� | Ũ��Ϊ0.2 mol/L��CH3COOH��Һ��Ũ��Ϊ0.1 mol/L��NaOH��Һ�������Ϻ�c��CH3COO -��-c��CH3COOH��=2[c��H+��-c��OH -��] | |
| C�� | ����Ũ��Һ������ˮϡ�ͣ�$\frac{c��C{H}_{3}COOH��}{{c}^{2}��{H}^{+}��}$�������� | |
| D�� | a mol/L CH3COOH��Һ��b mol/L NaOH��Һ�������ϣ�������Һ��c��Na+����c��CH3COO-������һ����a��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1 mol•L-1 | |
| B�� | һ������0.1 mol•L-1 | |
| C�� | ��Ϊǿ��һ������0.1 mol•L-1����Ϊ����һ��С��0.1 mol•L-1 | |
| D�� | ��Ϊǿ��һ����0.1 mol•L-1����Ϊ����һ����0.1 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ�е�KW��ͬ����ˮ�������c��H+������=��=��=�� | |
| B�� | ȡ�����ͬ����Һ�١��ڡ��۷ֱ����������۷�Ӧ������H2������������ | |
| C�� | ���������������Һ�ֱ�ϡ��100����������Һ��pH���ۣ��ܣ��ڣ��� | |
| D�� | �����£����ں͢۵������ϣ�c��CH3COO-��-c��Na+��=c��H+��-c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.05mol•L-1��H2SO4 | B�� | 0.1mol•L-1��KNO3 | ||
| C�� | 0.1mol•L-1��KOH | D�� | 0.1mol•L-1��NH4NO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
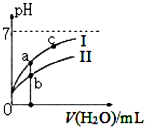 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����| CH3COOH | HClO | H2CO3 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.4��10-7Ka2=4.7��10-11 |
| A�� | ͼ��c��H+����c��R-����ֵ��a�㣾c�㣨HR����CH3COOH��HCIO�� | |
| B�� | pH��ͬ��������ҺŨ�ȹ�ϵ��c��CH3COONa��c��NaHC03��c��NaClO��c��Na2C03�� | |
| C�� | ͼ��a�������Ũ��С��b�������Ũ�� | |
| D�� | Ũ�Ⱦ�Ϊ0��l mol/L��CH3COONa��NaCIO�Ļ����Һ�У�c��OH-��=0��l mol/L-c��ClO-��+c��H+��+c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
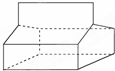 ����Ľṹ��ͼ��ʾ��
����Ľṹ��ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com