
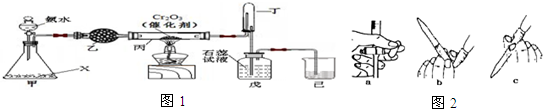
���� ��1������������Һ�IJ��������ж�����������
��2����װ�ò���Ҫ���ȼ���ͬʱ����������������˵������X���ڰ�ˮ�������ֲ���������
��3����װ��Ϊ����ܣ������Ǹ��������Ͱ����Ļ�����壻��װ�÷�����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��4����������������ԣ���ʹ��ɫʯ����Һ����жϣ�û�й۲쵽����˵����Һ�������ԣ������ɵ����������˷�Ӧ���ڱ��Ͷ�֮������ʢ����ˮ�Ȼ��Ƶĸ���ܻ�ʢ��Ũ�����ϴ��ƿ��ȥ����İ���ȷ������Һ�����ԣ�
��5�����ݼ�ʽ�ζ��ܵ�ʹ�÷��������жϣ��ζ�ǰ���ֵζ��ܼ��촦���������ݣ���ѡ���ų����ݵ���ȷ������b��
��� �⣺��1����ȡŨ��ˮҪ����Ͳ��ϡ��Ũ��ˮҪ���ձ�����������������ҺҪ��100mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����ͷ�ιܡ���Ͳ��
��2����װ�ò���Ҫ���ȼ���ͬʱ����������������˵������X���ڰ�ˮ�������ֲ�����������ù���Ϊ�������ƣ�
�ʴ�Ϊ���������ƣ�
��3����װ��Ϊ����ܣ������Ǹ��������Ͱ����Ļ�����壻��װ�÷�����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ�����������Ͱ����Ļ�����壻 4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��4����Ϊ����������ԣ���ʹ��ɫʯ����Һ��죬���Ե����й۲쵽��ɫʯ����Һ��죬˵�����Ƶ����û�й۲쵽����˵����Һ�������ԣ������ɵ�����������İ��������˷�Ӧ���ڱ��Ͷ�֮������ʢ����ˮ�Ȼ��Ƶĸ���ܻ�ʢ��Ũ�����ϴ��ƿ��ȥ����İ���ȷ������Һ�����ԣ�
�ʴ�Ϊ����ɫʯ����Һ��죻�����İ�����ʹ������Һ��һ�������ԣ��ڱ��Ͷ�֮������ʢ����ˮ�Ȼ��Ƶĸ���ܻ�ʢ��Ũ�����ϴ��ƿ��
��5����0.1mol•L-1��NaOH��Һ�ζ�ѡ���ʽ�ζ��ܣ�ͼ2��a��c��Ϊ��ʽ�ζ��ܣ��ζ�ǰ���ֵζ��ܼ��촦���������ݣ��ų����ݵ���ȷ������b��
�ʴ�Ϊ��b��
���� ���⿼����ʵ�����ư�����ԭ����װ���Լ�̽��������Ʊ������ʣ�ͬʱ������Ũ���ᡢ���������ʵ�����Ӧ���Լ��ζ��ܵ�ѡ����Ŀ�Ѷ��еȣ�


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ�� Cl2 | B�� | ���� Cl2 ���� O2 | ||
| C�� | ֻ�� O2 | D�� | ֻ�� H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ħ���ǻ�ѧ�ϳ��õ�һ�������� | |
| B�� | ��������ƽ��ȡ25.20 gNaCl | |
| C�� | ����һ�����ʵ���Ũ�ȵ���Һ������ʱ���ӿ̶��ᵼ��������ҺŨ��ƫС | |
| D�� | ij���ʺ���6.02��1023���������������Ŀ�������ʲ�һ����1 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Һ�ȣ�Cl2�� | B�� | ��ˮ��Cl2�� | C�� | Ư��Һ��NaClO�� | D�� | Ư��[Ca��ClO��2] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | ���鷽�� |
| A | CO32- | ����Ʒ�м����������ᣬ�����ɵ���ɫ��ζ������ͨ�����ʯ��ˮ�У��۲���Һ�Ƿ����� |
| B | SO42- | ����Ʒ���ȼ���ϡ�����ữ���ٵμ��Ȼ�����Һ���۲��Ƿ��а�ɫ�������� |
| C | Fe2+ | ȡ������Һ���Թ��У��������Ը��������Һ���۲���Һ��ɫ�Ƿ���ȥ |
| D | I- | ȡ������Һ���Թ��У�����������ˮ���ټ��������Һ���۲���Һ�Ƿ����ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
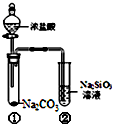
| A�� | ��Na2SiO3��Һ��ֱ�ӵ���������Һ���������� | |
| B�� | �Թܢ��з�Ӧ�����ӷ���ʽ�ǣ�Na2CO3+2H+�T2Na++CO2��+H2O | |
| C�� | ��ʵ�����֤�����ԣ����̼����� | |
| D�� | һ��ʱ����Թܢ����н���״�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
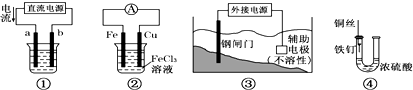
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �û��Һ��һ�����У�K+��NH4+��CO32-��SO42-�����ܺ�Cl- | |
| B�� | �û��Һ��һ�����У�NH4+��CO32-��SO42-�����ܺ�K+��Cl- | |
| C�� | �û��Һ��һ�����У�NH4+��CO32-��SO42-�����ܺ�Mg2+��K+��Cl- | |
| D�� | �û��Һ��һ�����У�NH4+��SO42-�����ܺ�Mg2+��K+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
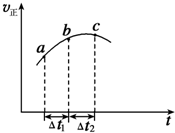 ����Ⱥ����ܱ�������ͨ��SO2��NO2һ��������ʹ��ӦSO2��g��+NO2��g��
����Ⱥ����ܱ�������ͨ��SO2��NO2һ��������ʹ��ӦSO2��g��+NO2��g��| A�� | ��Ӧ��C��ﵽƽ��״̬ | |
| B�� | ��Ӧ�������������������������� | |
| C�� | ��Ӧ��Ũ�ȣ�a�����b�� | |
| D�� | ��t1=��t2ʱ��SO2��ת���ʣ�a��b��С��b��c�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com