


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�������к��м��Ȳ��ӷ������� |
| B�������к��м����ӷ������� |
| C������ǰ�������в�����ˮ |
| D��ʵ��ǰ����δ��ȫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
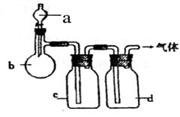
| ѡ�� | ���� | a | b | c | d |
| A | CO2 | ���� | CaCO3 | ����Na2CO3��Һ | Ũ���� |
| B | Cl2 | Ũ���� | MnO2 | NaOH��Һ | Ũ���� |
| C | NH3 | ����NH4Cl��Һ | ��ʯ�� | H2O | ����NaOH |
| D | NO | ϡ���� | ͭм | H2O | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
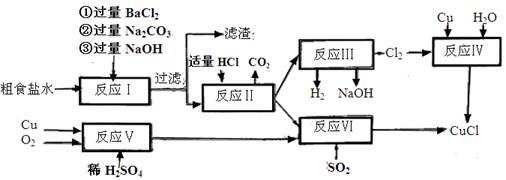
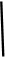 ��
���鿴�𰸺ͽ���>>
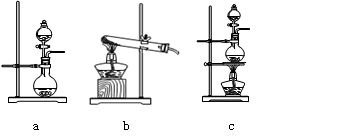
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
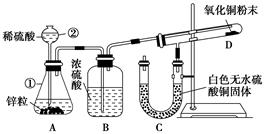
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2CaSO4+2Cl2��+2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�
2CaSO4+2Cl2��+2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�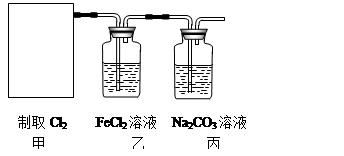
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
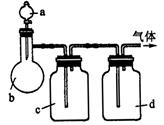
| ���� | a | b | c | d |
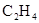 | �Ҵ� | Ũ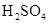 | 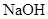 ��Һ ��Һ | Ũ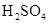 |
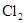 | Ũ���� | 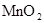 | 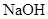 ��Һ ��Һ | Ũ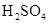 |
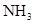 | ����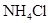 ��Һ ��Һ | ��ʯ�� | 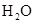 | ����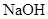 |
| NO | ϡ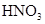 | ͭм | 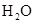 | 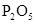 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
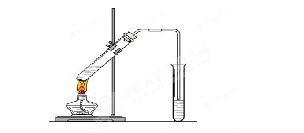
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
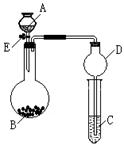
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com