
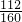 ����Ʒ����Ԫ�ص�����������
����Ʒ����Ԫ�ص�����������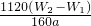 ��100%��
��100%��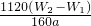 ��100%��
��100%�� c��b��10-3
c��b��10-3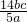 ���ʴ�Ϊ��
���ʴ�Ϊ��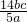 ��
��

 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��.��ˮ���Ȼ�����Һ����ѧ��ѧʵ���еij����Լ���Ũ�Ƚ�Сʱ����Һ���ʻ�ɫ��ijͬѧ��ϡ��ˮ��ϡFeCl2��Һ��ϣ�������Һ�Ի�ɫ��Ϊ̽����ˮ��FeCl2��Һ����ܷ�����Ӧ����ͬѧ���������ʵ�鷽����
| ���� | ʵ������ | ʵ����� | |
| ����1 | ȡ���������Һ������NaOH��Һ���� | �õ����ɫ���� | �����˻�ѧ��Ӧ |
| ����2 | ȡ���������Һ���������Ȼ�̼��Һ���� | �л���ʳȻ�ɫ | δ������ѧ��Ӧ |
| ����3 | ȡ���������Һ��������۵⻯����Һ���� | ��Һ����ɫ | δ������ѧ��Ӧ |
��ش��������⣺
��1������2�Ľ��۲���������������
��2������3�Ľ������Բ����������ܷ�����Ӧ�����ӷ���ʽ
��3�����������һ����ʵ�鷽�������������������������ۡ������ж���ˮ��FeCl2��Һ�Ƿ�Ӧ��
��
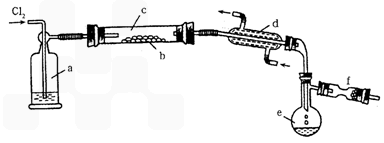
 ��.S2Cl2��һ���ӷ���Һ�壨�۵㣺��76�棬�е㣺138�棩��������ˮ����ˮ�ⷴӦ����������H2S��SO2��H2SO3��H2SO4�����ʡ����������������ڵ�����ͨ��������������S2Cl2����ͼ��ʵ������S��Cl2�Ʊ�S2Cl2��װ�ã��г�װ�á�����װ�þ�����ȥ����
��.S2Cl2��һ���ӷ���Һ�壨�۵㣺��76�棬�е㣺138�棩��������ˮ����ˮ�ⷴӦ����������H2S��SO2��H2SO3��H2SO4�����ʡ����������������ڵ�����ͨ��������������S2Cl2����ͼ��ʵ������S��Cl2�Ʊ�S2Cl2��װ�ã��г�װ�á�����װ�þ�����ȥ����
��1��װ��a��Ӧ���Լ�Ϊ__________��װ��d��������_________������������_________��
��2����ʵ��IJ���˳��ӦΪ__________������ű�ʾ����
�ټ���װ��c ��ͨ��Cl2 ��ͨ����ˮ ��ֹͣͨCl2 ��ֹͣ����װ��c
��3����S2Cl2��ˮ���������ͨ����ˮ�У����۲쵽_________���������֤��ˮ����������������ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
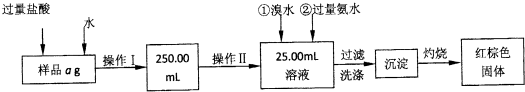
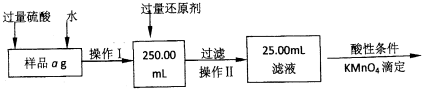
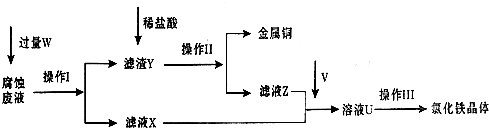
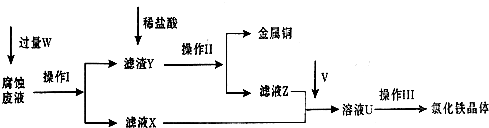
��FeCl3��Һʴ��ͭ�������·��Ĺ����У���Һ��������Դ���յĹ��̼������£���ش���������:

��1����д��FeCl3��Һ��ͭ��Ӧ�����ӷ���ʽ�� �������������Լ�W�� ��
��2��������֮ǰ��ü�������ϡ���ᣬijͬѧ��10mol• L-1 ��Ũ��������250mL 1mol• L-1 ��ϡ���ᣬ�������й�ʵ�顣
����Ҫ��ȡŨ���� mL��
�����Ƹ�ϡ����ʱ����Ͳ���ձ����������⣬�������õ��������� �� ��
������֪Ũ�ȵ�����������Һ�ζ�ϡ���ᣬ�ζ��������۾�Ӧע�� ��ʵ��ʱδ�ñ�Һ��ϴ�ζ��ܣ������ƿ�д���Һ��Ũ�� ʵ��Ũ�ȡ������������������=������
��3��������ǰ��Ҫͨ������V����д��ʵ������ȡ����V�Ļ�ѧ����ʽ: ���÷�Ӧ�� ����������
��4��������Ӧ��HCl������Χ�н��У���ԭ���� ��
��5����ͨ���V�������㣬������Ƶõ��Ȼ������岻������������Ϊ��ҺU�к������� ������ƺ�����ʵ�飬��֤��ҺU�еijɷ֣����ж�ͨ���V�����Ƿ��� ����Ҫ˵��ʵ�鲽�衢����ͽ��ۣ���
��ѡ����Լ�:����KMnO4��Һ��KSCN��Һ����ˮ��
��6�������Ȼ�����Һ�м���һ����ʯ��ˮ��������ҺpH���ɵú��ɫ�������ù����е�����Һ��pHΪ5�����������Ũ��Ϊ ������֪��Ksp[Fe(OH)3]= 4.0��10-38��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com