
��10�֣�
���п�ͼ�漰������������Ԫ���У���һ��Ԫ���⣬�����Ϊ1��18��Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ��ȫ����
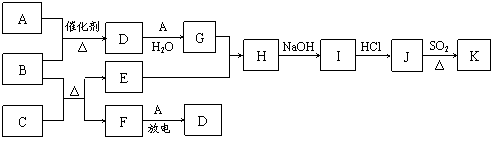
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��J��K��ͬ�ֽ������Ȼ����KΪ��ɫ������д��SO2��ԭJ����K�����ӷ���ʽ ��
��3������β���г�����D��B�ڼ��Ⱥʹ������ڵ�������������D����Ⱦ����֪��
![]() ��4NH3(g)��5O2(g) 4NO(g)��6H2O(g) ��H����905 kJ��mol��1
��4NH3(g)��5O2(g) 4NO(g)��6H2O(g) ��H����905 kJ��mol��1
��4NH3(g)��3O2(g)![]() 2N2(g)��6H2O(g) ��H����1268 kJ��mol��1
2N2(g)��6H2O(g) ��H����1268 kJ��mol��1
��B��D��Ӧ���Ȼ�ѧ����ʽΪ ��
��4��������Ϊ�������滯ѧ���о��ɹ���ʹB��D�ķ�Ӧ�ڴ����������ʱ��Ч�ʴ����ߣ��Ӷ�ʹ��Ⱦ��D��ת���ʴ����ߡ�����Ӧ�û�ѧ�������۶Դ˹۵�������ۣ� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ����и���ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��10�֣����п�ͼ�漰������������Ԫ���У���һ��Ԫ���⣬�����Ϊ1��18��Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ��ȫ����
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��J��K��ͬ�ֽ������Ȼ����KΪ��ɫ������д��SO2��ԭJ����K�����ӷ���ʽ ��
��3������β���г�����D��B�ڼ��Ⱥʹ������ڵ�������������D����Ⱦ����֪��
��4NH3(g)��5O2(g)  4NO(g)��6H2O(g) ��H����905 kJ��mol��1
4NO(g)��6H2O(g) ��H����905 kJ��mol��1
��4NH3(g)��3O2(g) 2N2(g)��6H2O(g) ��H����1268 kJ��mol��1
2N2(g)��6H2O(g) ��H����1268 kJ��mol��1
��B��D��Ӧ���Ȼ�ѧ����ʽΪ ��
��4��������Ϊ�������滯ѧ���о��ɹ���ʹB��D�ķ�Ӧ�ڴ����������ʱ��Ч�ʴ����ߣ��Ӷ�ʹ��Ⱦ��D��ת���ʴ����ߡ�����Ӧ�û�ѧ�������۶Դ˹۵�������ۣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�����ѧ����10�½���ϰ��ѧ�Ծ����������� ���ͣ������
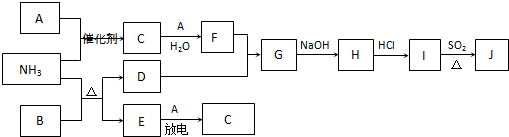
��12�֣����п�ͼ�漰������������Ԫ���У���һ��Ԫ���⣬�����Ϊ1��18��Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ��ȫ����
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��J��K��ͬ�ֽ������Ȼ����KΪ��ɫ������д��SO2��ԭJ����K�����ӷ���ʽ ��
��3������β���г�����D��B��D�ڼ��Ⱥʹ������ڵ������£�������Ӧ�������ֶԿ�������Ⱦ�����ʡ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��������Ϊ�������滯ѧ���о��ɹ���ʹB��D�ķ�Ӧ�ڴ����������ʱ��Ч�ʴ����ߣ��Ӷ�ʹ��Ⱦ��D��ת���ʴ����ߡ�����Ӧ�û�ѧ�������۶Դ˹۵�������ۣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ����10�½���ϰ��ѧ�Ծ��������棩 ���ͣ������
��12�֣����п�ͼ�漰������������Ԫ���У���һ��Ԫ���⣬�����Ϊ1��18��Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ��ȫ����

��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��J��K��ͬ�ֽ������Ȼ����KΪ��ɫ������д��SO2��ԭJ����K�����ӷ���ʽ ��
��3������β���г�����D��B��D�ڼ��Ⱥʹ������ڵ������£�������Ӧ�������ֶԿ�������Ⱦ�����ʡ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��������Ϊ�������滯ѧ���о��ɹ���ʹB��D�ķ�Ӧ�ڴ����������ʱ��Ч�ʴ����ߣ��Ӷ�ʹ��Ⱦ��D��ת���ʴ����ߡ�����Ӧ�û�ѧ�������۶Դ˹۵�������ۣ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com