

| V3-(V2-V1) |
| 22400m |
| V3-(V2-V1) |
| 22400m |
| V3-(V2-V1) |
| 22400 |
| V3-(V2-V1) |
| 22400m |
| V3-(V2-V1) |
| 22400m |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ͼ6 ͼ7
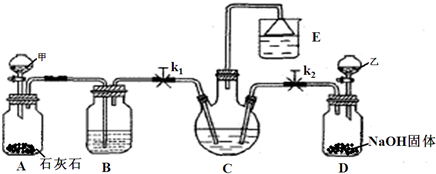
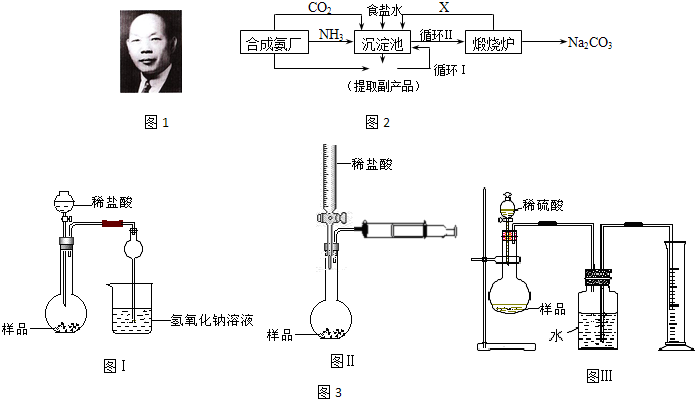
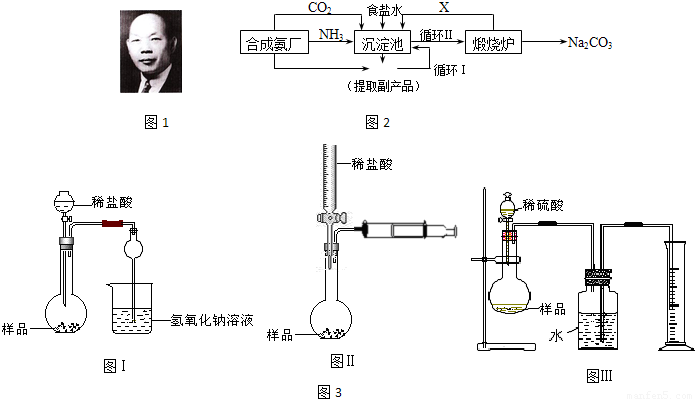
(1)������������ķ�����________________������Ʒ��һ����;Ϊ____________________��
(2)�������з����Ļ�ѧ��Ӧ����ʽ��________________________________________��
(3)д������������X���ʵķ���ʽ________________________________��
(4)ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%���ϣ���Ҫ�������_________�������������еı�ţ���ѭ�����ӳ�������ȡ�������IJ�����_____________________________��
(5)Ϊ�����Ʒ̼�������Ƿ����Ȼ��ƣ���ȡ������������ˮ���ٵμ�________________��
(6)��ĸҺ��ͨ����������ϸСʳ�ο�������ȴ��������Ʒ��ͨ������������______________��
a.����![]() ��Ũ�ȣ�ʹNH4Cl���������
��Ũ�ȣ�ʹNH4Cl���������
b.ʹNaHCO3���������
c.ʹNaHCO3ת��ΪNa2CO3�����������NH4Cl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ��Ǩ�и߿���ѧ����Ծ��������������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com