
����˵����ȷ����
A����H<0����S>0�ķ�Ӧ���¶ȵ�ʱ�����Է�����
B��NH4HCO3(s)===NH3(g)��H2O(g)��CO2(g)����H����185.57 kJ/mol���Է����У�ԭ������ϵ���Է�������Ҷ����ӵķ���ת�������
C����Ϊ�ʱ���ر䶼�뷴Ӧ���Է����йأ�����ʱ���ر�����Ե�����Ϊ��Ӧ�Է��Ե��о�
D������������������������£�ʹ�ô��������Ըı仯ѧ��Ӧ���еķ���
 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�¶��£���Cl2ͨ��NaOH��Һ�У���Ӧ�õ�NaCl��NaClO��NaClO3�Ļ����Һ�����ⶨClO����ClO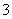 �����ʵ���Ũ��֮��Ϊ1��3����˷�Ӧ�б���ԭ����Ԫ���뱻��������Ԫ��ԭ�ӵ����ʵ���֮����(����
�����ʵ���Ũ��֮��Ϊ1��3����˷�Ӧ�б���ԭ����Ԫ���뱻��������Ԫ��ԭ�ӵ����ʵ���֮����(����
A��21��5 B��11��3
C��4��1 D��3��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������þ��ҽҩ����������ҵӦ�ù㷺������þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3)Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�21���ͽ�������Ȩ����

(1)MgCO3��ϡ���ᷴӦ�����ӷ���ʽΪ________________________________________________________________________
________________________________________________________________________��
(2)����H2O2����ʱ��������Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________
________________________________________________________________________��
(3)����2�ijɷ��ǡ�Fe(OH)3��(�ѧʽ)��
(4)���չ��̴������·�Ӧ��
2MgSO4��C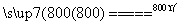 2MgO��2SO2����CO2��
2MgO��2SO2����CO2��
MgSO4��C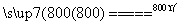 MgO��SO2����CO��
MgO��SO2����CO��
MgSO4��3C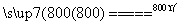 MgO��S����3CO��
MgO��S����3CO��
������ͼװ�ö����ղ�����������зֲ����ջ��ռ���

��D���ռ�����������ǡ�CO��(�ѧʽ)��
��B��ʢ�ŵ���Һ�����ǡ�d��(����ĸ)��
a��NaOH��Һ b��Na2CO3��Һ
c��ϡ���� d��KMnO4��Һ
��A�еõ��ĵ���ɫ���������ȵ�NaOH��Һ��Ӧ��������Ԫ�����̬Ϊ��4��д���÷�Ӧ�����ӷ���ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ֵ�����ɵĺϽ�A��Ϊ������ɷ֣��ô˺Ͻ����һϵ��ʵ�飬����̼�������Ӧ����������ͼ��ʾ��

��д���пհף�
(1)A����������Ϊ��Fe��Al��Si��C��(д��ѧʽ)��
(2)д��A�е�ij�������ռ���Һ��Ӧ�����ӷ���ʽ
________________________________________________________________________
________________________________________________________________________��
(3)���ó��ι���dz��ɫ��ҺH�м���NaOH��Һ������������________________________________________________________________________
________________________________________________________________________��
(4)д������F�����ᷴӦ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��2H2(g)��O2(g)===2H2O(l) ��H����571.6kJ��mol��1
CH4(g)��2O2(g)===CO2(g)��2H2O(l) ��H����890kJ��mol��1 ����H2��CH4�Ļ������112L(��״��)��ʹ����ȫȼ������CO2��H2O(l)����ʵ���÷�Ӧ����3695kJ����ԭ���������H2��CH4�����ʵ���֮����
A��1�U1���� B��1�U3���� C��1�U4���� D��2�U3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У���֤��ij������������ʵ���
A���ۻ�ʱ���ܵ��� B���������ӻ������ǹ��ۻ�����
C��ˮ��Һ�ĵ��������ܲ�
D����Һ�д��ڵ���ƽ�⣬����������Ӻ�δ����ķ��ӹ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ũ�Ⱦ�Ϊ0.5mol/L��������Һ����Na2CO3����NaHCO3����HCl����NH3��H2O��
(1)������Һ�У��ɷ���ˮ�����________(����ţ���ͬ)��
(2)������Һ�У��������������Ʒ�Ӧ�����ܺ����ᷴӦ����Һ������Ũ���ɴ�С��˳��Ϊ________________________________________��
(3)����Һ���м��������Ȼ�粒��壬��ʱc(NH4��)/c(OH��)��ֵ________(���������С���������䡱)��
(4)�����ۺܵ͢���Һ��Ϻ���Һǡ�ó����ԣ�����ǰ�۵����________�ܵ����(����ڡ�����С�ڡ��������ڡ�)����ʱ��Һ������Ũ���ɴ�С��˳����______________________��
(5)ȡ10mL��Һ�ۣ���ˮϡ�͵�500mL�������Һ����ˮ�������c(H��)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ǻ�ѧ�о��Ļ��������й��ڸ�ʵ��װ�õ�������ȷ���ǣ� ��

A��װ�âٳ����ڷ��뻥�����ܵ�Һ������
B��װ�âڿ���������NH3��HCl���壬����ֹ����
C��װ�âۿ������ռ�H2��CO2��Cl2��NH3������
D��װ�âܿ����ڸ���ռ��Ȼ��⣬�����ն�����Ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���¶��µĶ����ܱ������У�������������������ʱ��仯ʱ��������Ӧ��
A (s)+2B (g)  C (g)+D (g)һ���Ѵﵽƽ��״̬���ǣ�
C (g)+D (g)һ���Ѵﵽƽ��״̬���ǣ�
A����������ѹǿ ���� B�����������ܶ�
C�������������ʵ��� ���� D�����������C��D�����ʵ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com