
(1)CH4(g )+2O2(g )=CO2(g )+2H2O(g ) ¦¤H=-802.3kJ/mol
øĆČČ»Æѧ·“Ó¦·½³ĢŹ½µÄŅāŅåŹĒ_____________________________________”£
(2)ŅŃÖŖ2gŅŅ“¼ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®·Å³öQ kJµÄČČĮ棬Š“³ö±ķŹ¾ŅŅ“¼Č¼ÉÕČȵÄČČ»Æѧ·½
³ĢŹ½£ŗ____________________________________________________________.
(3)ŅŃÖŖ²šæŖ1mol H-H¼ü£¬1mol N-H¼ü£¬1mol 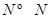 ¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436kJ”¢391KJ”¢946kJ£¬ŌņN2ÓėH2·“Ӧɜ³É1mol NH3(g)µÄČČ»Æѧ·½³ĢŹ½ŹĒ___________________.
¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436kJ”¢391KJ”¢946kJ£¬ŌņN2ÓėH2·“Ӧɜ³É1mol NH3(g)µÄČČ»Æѧ·½³ĢŹ½ŹĒ___________________.
(4)ŅĄ¾ŻøĒĖ¹¶ØĀÉæÉŅŌ¶ŌijŠ©ÄŃŅŌĶعżŹµŃéÖ±½Ó²ā¶ØµÄ»Æѧ·“Ó¦µÄģŹ±ä½ųŠŠĶĘĖć”£
ŅŃÖŖ£ŗC(ŹÆÄ«£¬s)+O2(g)=CO2(g) ¦¤H=-393.5kJ/mol ¢Ł
2H2(g)+O2(g)=2H2O(l) ¦¤H=-571.6kJ/mol ¢Ś
2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l) ¦¤H=-2599kJ/mol ¢Ū
øł¾ŻøĒĖ¹¶ØĀÉ£¬¼ĘĖć298KŹ±ÓÉC£ØŹÆÄ«£¬s£©ŗĶH2(g)Éś³É1mol C2H2(g)·“Ó¦µÄģŹ±ä£ŗ
____________________________.
£Ø1£©1mol¼×ĶéĘųĢåŗĶ2molµÄŃõĘųĶźČ«·“Ӧɜ³É1mol¶žŃõ»ÆĢ¼ŗĶ2molĖ®ÕōĘųŹ±·Å³ö802.3KJµÄČČĮ攣£Ø2£©C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ; ¦¤H=-23QKJØMmol (3)( 1ØM2) N2(g)+( 3ØM2) H2(g)=NH3(g) ; ¦¤H=-46KJØMmol (4)+226.7KJØMmol”£
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øĆČČ»Æѧ·“Ó¦·½³ĢŹ½µÄŅāŅåŹĒ1mol¼×ĶéĘųĢåŗĶ2molµÄŃõĘųĶźČ«·“Ӧɜ³É1mol¶žŃõ»ÆĢ¼ŗĶ2molĖ®ÕōĘųŹ±·Å³öČČĮæ802.3KJ”££Ø2£©ŅŅ“¼µÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ46.1molŅŅ“¼µÄÖŹĮæĪŖ46g”£ŅņĪŖ2gŅŅ“¼Č¼ÉÕÉś³ÉŅŗĢ¬Ė®·ÅČČQ kJ£¬ĖłŅŌ46gŅŅ“¼Č¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³öµÄČČĮæĪŖ23 Q kJ£¬ĖłŅŌĘäČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗC2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ; ¦¤H=-23QKJØMmol”£·“Ó¦ČČŹĒ¶ĻĮŃ»Æѧ¼üĪüŹÕµÄÄÜĮæÓėŠĪѧ¼üĖłŹĶ·ÅµÄÄÜĮæ²ī”£ĖłŅŌN2ÓėH2·“Ӧɜ³É1mol NH3(g)µÄ·“Ó¦ČČĪŖ( 1ØM2)”Į946+( 3ØM2) ”Į436-3”Į391=-46.¹ŹøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ( 1ØM2) N2(g)+( 3ØM2) H2(g)=NH3(g) ; ¦¤H="-46KJØMmol" (4) ¢Ł”Į2+£Ø1ØM2 £©”Į¢Ś-£Ø1ØM2 £©”Į¢ŪµĆ¦¤H=2”Į(-393.5)+£Ø1ØM2 £©”Į(-571.6)+£Ø1ØM2 £©”Į2599=+226.7KJØMmol.”£øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ2C(ŹÆÄ«£¬s)+H2(g)=C2H2(g ) ; ¦¤H=+226.7KJØMmol.
æ¼µć£ŗæ¼²éøĒĖ¹¶ØĀÉÓ¦ÓĆ”¢ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“ŅāŅ唢¼°Óė»Æѧ¼üµÄ¹ŲĻµµČµÄÖŖŹ¶”£


 ¾ŁŅ»·“ȿʌĩ°Ł·Ö³å“Ģ¾ķĻµĮŠ“š°ø
¾ŁŅ»·“ȿʌĩ°Ł·Ö³å“Ģ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¢ńŌŚ“߻ƼĮ×÷ÓĆĻĀ£¬CO2ŗĶH2æÉŅŌÖĘČ”¼×“¼”£ÓĆ¹¤Ņµ·ĻĘųÖŠµÄ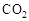 æÉÖĘČ”¼×“¼£¬Ęä·“Ó¦ĪŖ£ŗCO2+3H2
æÉÖĘČ”¼×“¼£¬Ęä·“Ó¦ĪŖ£ŗCO2+3H2 CH3OH+H2O ³£ĪĀ³£Ń¹ĻĀŅŃÖŖĻĀĮŠ·“Ó¦µÄÄÜĮæ±ä»ÆČēĶ¼Ź¾£ŗ
CH3OH+H2O ³£ĪĀ³£Ń¹ĻĀŅŃÖŖĻĀĮŠ·“Ó¦µÄÄÜĮæ±ä»ÆČēĶ¼Ź¾£ŗ
Š“³öÓɶžŃõ»ÆĢ¼ŗĶĒāĘųÖʱø¼×“¼µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
¢ņÅšĒā»ÆÄĘ£ØNaBH4£©ŹĒÓŠ»śŗĻ³ÉÖŠµÄÖŲŅŖ»¹Ō¼Į”£×īŠĀŃŠ¾æ·¢ĻÖ£¬ŅŌNaBH4ŗĶH2O2ĪŖŌĮĻ£¬NaOHČÜŅŗ×÷µē½āÖŹČÜŅŗ£¬æÉŅŌÉč¼Ę³ÉČ«ŅŗĮ÷µē³Ų£¬Ę乤×÷ŌĄķČēĶ¼ĖłŹ¾£¬¼ŁÉčµē³Ų¹¤×÷Ē°×óÓŅĮ½²ŪČÜŅŗµÄĢå»żø÷ĪŖ1L£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©µē¼«bĪŖ £ØĢī”°Õż¼«”±»ņ”°øŗ¼«”±£©£¬µē¼«aÉĻ·¢Éś·“Ó¦µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©µē³Ų¹¤×÷Ź±£¬Na+Ļņ ¼«£ØĢī”°a”±»ņ”°b”±£©ŅĘ¶Æ£¬µ±×ó²Ū²śÉś0£®0125molBO2”ŖĄė×ÓŹ±£¬ÓŅ²ŪČÜŅŗpH=
£Ø3£©ÓĆøƵē³Ųµē½āŅ»¶ØÅØ¶ČµÄCuSO4ČÜŅŗÖĮĪŽÉ«ŗó¼ĢŠųµē½āŅ»¶ĪŹ±¼ä”£¶ĻæŖµēĀ·£¬ĻņČÜŅŗÖŠ¼ÓČė0£®1molCu(OH)2£¬ČÜŅŗ»Öø“µ½µē½āÖ®Ē°×“Ģ¬£¬Ōņµē½ā¹ż³ĢÖŠ×ŖŅʵē×ÓŹżÄæĪŖ_________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©°ŃĆŗ×÷ĪŖČ¼ĮĻæÉĶعżĻĀĮŠĮ½ÖÖĶ¾¾¶£ŗ
Ķ¾¾¶I£ŗC(s) +O2 (g)=CO2(g) ”÷H1<0 ¢Ł
Ķ¾¾¶II£ŗĻČÖĘ³ÉĖ®ĆŗĘų£ŗC(s) +H2O(g)=CO(g)+H2(g) ”÷H2>0 ¢Ś
ŌŁČ¼ÉÕĖ®ĆŗĘų£ŗ2CO(g)+O2 (g)=2CO2(g) ”÷H3<0 ¢Ū
2H2(g)+O2 (g)=2H2O(g) ”÷H4<0 ¢Ü
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Ķ¾¾¶I·Å³öµÄČČĮæ ( Ģī”°“óÓŚ”±”°µČÓŚ”±»ņ”°Š”ÓŚ”±) Ķ¾¾¶II·Å³öµÄČČĮ攣
£Ø2£©”÷H1”¢”÷H2”¢”÷H3”¢”÷H4µÄŹżŃ§¹ŲĻµŹ½ŹĒ ”£
£Ø3£©12gĢæ·ŪŌŚŃõĘųÖŠ²»ĶźČ«Č¼ÉÕÉś³ÉŅ»Ńõ»ÆĢ¼£¬·Å³ö110.35kJČČĮ攣ĘäČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ĆŗĢæ×÷ĪŖČ¼ĮĻ²ÉÓĆĶ¾¾¶IIµÄÓŵćÓŠ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĒāŃõĮ½ÖÖŌŖĖŲŠĪ³ÉµÄ³£¼ūĪļÖŹÓŠH2OÓėH2O2£¬ŌŚŅ»¶ØĢõ¼žĻĀ¾łæÉ·Ö½ā”£
£Ø1£©ŅŃÖŖ£ŗ
| »Æѧ¼ü | ¶ĻæŖ1mol»Æѧ¼üĖłŠčµÄÄÜĮæ£ØkJ£© |
| H”ŖH | 436 |
| O”ŖH | 463 |
| O=O | 498 |
| ŹµŃ鱹ŗÅ | ·“Ó¦Īļ | “߻ƼĮ | |
| a | 50 mL 5% H2O2ČÜŅŗ | | 1 mL 0.1 mol”¤L-1 FeCl3ČÜŅŗ |
| b | 50 mL 5% H2O2ČÜŅŗ | ÉŁĮæÅØŃĪĖį | 1 mL 0.1 mol”¤L-1 FeCl3ČÜŅŗ |
| c | 50 mL 5% H2O2ČÜŅŗ | ÉŁĮæÅØNaOHČÜŅŗ | 1 mL 0.1 mol”¤L-1 FeCl3ČÜŅŗ |
| d | 50 mL 5% H2O2ČÜŅŗ | | MnO2 |
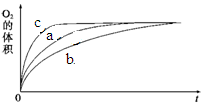
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŹ©ŅŌ¼õÉŁÄÜŌ“ĄĖ·ŃŗĶ½µµĶ·ĻĘųÅÅ·ÅĪŖ»ł±¾ÄŚČŻµÄ½ŚÄܼõÅÅÕž²ß£¬ŹĒÓ¦¶ŌČ«ĒņĘųŗņĪŹĢā”¢½ØÉč׏Ō“½ŚŌ¼ŠĶ”¢»·¾³ÓŃŗĆŠĶÉē»įµÄ±ŲȻєŌń”£»Æ¹¤ŠŠŅµµÄ·¢Õ¹±ŲŠė·ūŗĻ¹ś¼Ņ½ŚÄܼõÅŵÄ×ÜĢåŅŖĒó”£ŹŌŌĖÓĆĖłŃ§ÖŖŹ¶£¬½ā¾öĻĀĮŠĪŹĢā£ŗ
¢ÅŅŃÖŖij·“Ó¦µÄĘ½ŗā±ķ“ļŹ½ĪŖ£ŗ
ĖüĖł¶ŌÓ¦µÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ£ŗ
¢ĘĄūÓĆĖ®ĆŗĘųŗĻ³É¶ž¼×ĆѵÄČż²½·“Ó¦ČēĻĀ£ŗ
¢Ł2H2£Øg£©+CO£Øg£© CH3OH£Øg£©£»¦¤H=£90.8kJ”¤mol
CH3OH£Øg£©£»¦¤H=£90.8kJ”¤mol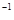
¢Ś2CH3OH£Øg£© CH3OCH3£Øg£©+H2O£Øg£©£»¦¤H=£23.5kJ”¤mol
CH3OCH3£Øg£©+H2O£Øg£©£»¦¤H=£23.5kJ”¤mol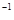
¢ŪCO£Øg£©+H2O£Øg£© CO2£Øg£©+H2£Øg£©£»¦¤H=£41.3kJ”¤mol
CO2£Øg£©+H2£Øg£©£»¦¤H=£41.3kJ”¤mol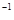
×Ü·“Ó¦£ŗ3H2£Øg£©+3CO£Øg£© CH3OCH3£Øg£©+CO2£Øg£©µÄ¦¤H=__________
CH3OCH3£Øg£©+CO2£Øg£©µÄ¦¤H=__________
(3)Ćŗ»Æ¹¤Ķس£ĶعżŃŠ¾æ²»Ķ¬ĪĀ¶ČĻĀĘ½ŗā³£ŹżŅŌ½ā¾öø÷ÖÖŹµ¼ŹĪŹĢā”£ŅŃÖŖµČĢå»żµÄŅ»Ńõ»ÆĢ¼ŗĶĖ®ÕōĘų½ųČė·“Ó¦Ę÷Ź±£¬»į·¢ÉśČēĻĀ·“Ó¦£ŗCO£Øg£©+H2O£Øg£© H2£Øg£©+CO2£Øg£©£¬øĆ·“Ó¦Ę½ŗā³£ŹżĖęĪĀ¶ČµÄ±ä»ÆČēĻĀ±ķĖłŹ¾£ŗ
H2£Øg£©+CO2£Øg£©£¬øĆ·“Ó¦Ę½ŗā³£ŹżĖęĪĀ¶ČµÄ±ä»ÆČēĻĀ±ķĖłŹ¾£ŗ
| ĪĀ¶Č/”ę | 400 | 500 | 800 |
| Ę½ŗā³£ŹżK | 9.94 | 9 | 1 |
 2NO2£Øg£©”÷H>0£¬ŌŚĪĀ¶ČĪŖT1”¢T2Ź±£¬Ę½ŗāĢåĻµÖŠNO2µÄĢå»ż·ÖŹżĖęŃ¹Ēæ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ”£
2NO2£Øg£©”÷H>0£¬ŌŚĪĀ¶ČĪŖT1”¢T2Ź±£¬Ę½ŗāĢåĻµÖŠNO2µÄĢå»ż·ÖŹżĖęŃ¹Ēæ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ”£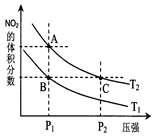
 CH3OH(g)+H2O(g)”÷H=£49.0kJ”¤mol£1”£ĻÖŌŚĪĀ¶Č”¢ČŻ»żĻąĶ¬µÄ3øöĆܱÕČŻĘ÷ÖŠ£¬°“²»Ķ¬·½Ź½Ķ¶Čė·“Ó¦Īļ£¬±£³ÖŗćĪĀ”¢ŗćČŻ£¬²āµĆ·“Ó¦“ļµ½Ę½ŗāŹ±µÄÓŠ¹ŲŹż¾ŻČēĻĀ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
CH3OH(g)+H2O(g)”÷H=£49.0kJ”¤mol£1”£ĻÖŌŚĪĀ¶Č”¢ČŻ»żĻąĶ¬µÄ3øöĆܱÕČŻĘ÷ÖŠ£¬°“²»Ķ¬·½Ź½Ķ¶Čė·“Ó¦Īļ£¬±£³ÖŗćĪĀ”¢ŗćČŻ£¬²āµĆ·“Ó¦“ļµ½Ę½ŗāŹ±µÄÓŠ¹ŲŹż¾ŻČēĻĀ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ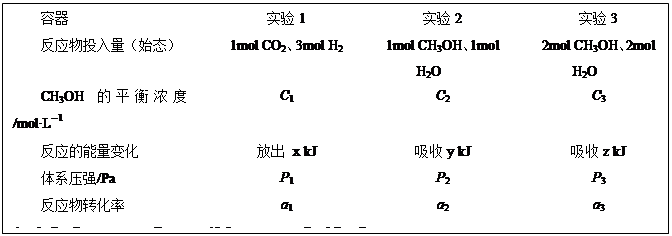
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½šŹōĪŁÓĆĶ¾¹ć·ŗ£¬Ö÷ŅŖÓĆÓŚÖĘŌģÓ²ÖŹ»ņÄĶøßĪĀµÄŗĻ½š£¬ŅŌ¼°µĘÅŻµÄµĘĖ攣øßĪĀĻĀ£¬ŌŚĆܱÕČŻĘ÷ÖŠÓĆH2»¹ŌWO3æɵƵ½½šŹōĪŁ£¬Ęä×Ü·“Ó¦ĪŖ£ŗ
WO3 (s) + 3H2 (g) W (s) + 3H2O (g)
W (s) + 3H2O (g)
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ÅÉĻŹö·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½ĪŖ___________________________”£
¢ĘijĪĀ¶ČĻĀ·“Ó¦“ļĘ½ŗāŹ±£¬H2ÓėĖ®ÕōĘųµÄĢå»ż±ČĪŖ2:3£¬ŌņH2µÄĘ½ŗā×Ŗ»ÆĀŹĪŖ_____________________£»ĖęĪĀ¶ČµÄÉżøߣ¬H2ÓėĖ®ÕōĘųµÄĢå»ż±Č¼õŠ”£¬ŌņøĆ·“Ó¦ĪŖ·“Ó¦_____________________£ØĢī”°ĪüČČ”±»ņ”°·ÅČČ”±£©”£
¢ĒÉĻŹö×Ü·“Ó¦¹ż³Ģ“óÖĀ·ÖĪŖČżøö½×¶Ī£¬ø÷½×¶ĪÖ÷ŅŖ³É·ÖÓėĪĀ¶ČµÄ¹ŲĻµČēĻĀ±ķĖłŹ¾£ŗ
| ĪĀ¶Č | 25”ę ~ 550”ę ~ 600”ę ~ 700”ę |
| Ö÷ŅŖ³É·Ż | WO3 W2O5 WO2 W |
 W (s) + 2H2O (g)£»¦¤H £½ +66.0 kJ”¤mol£1
W (s) + 2H2O (g)£»¦¤H £½ +66.0 kJ”¤mol£1  W (s) + 2H2O (g)£»¦¤H £½ £137.9 kJ”¤mol£1
W (s) + 2H2O (g)£»¦¤H £½ £137.9 kJ”¤mol£1  WO2 (g) µÄ¦¤H £½ ______________________”£
WO2 (g) µÄ¦¤H £½ ______________________”£ WI4 (g)”£ĻĀĮŠĖµ·ØÕżČ·µÄÓŠ____________”£
WI4 (g)”£ĻĀĮŠĖµ·ØÕżČ·µÄÓŠ____________”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌĖÓĆ»Æѧ·“Ó¦ŌĄķŃŠ¾æµŖ”¢Įņ”¢ĀČ”¢µāµČµ„ÖŹ¼°Ęä»ÆŗĻĪļµÄ·“Ó¦ÓŠÖŲŅŖŅāŅå
£Ø1£©ĮņĖįÉś²śÖŠ£¬SO2“ß»ÆŃõ»ÆÉś³ÉSO3£» 2SO2£Øg£©+O2£Øg£©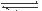 2SO3£Øg£©£¬»ģŗĻĢåĻµÖŠSO3 µÄ°Ł·Öŗ¬ĮæŗĶĪĀ¶ČµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ØĒśĻßÉĻČĪŗĪŅ»µć¶¼±ķŹ¾Ę½ŗāדĢ¬£©£®øł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£¬
2SO3£Øg£©£¬»ģŗĻĢåĻµÖŠSO3 µÄ°Ł·Öŗ¬ĮæŗĶĪĀ¶ČµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ØĒśĻßÉĻČĪŗĪŅ»µć¶¼±ķŹ¾Ę½ŗāדĢ¬£©£®øł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£¬
¢Ł2SO2£Øg£©+O2£Øg£© 2SO3£Øg£©µÄ”÷H 0
2SO3£Øg£©µÄ”÷H 0
£ØĢī”°>”±»ņ”°<”±£©£ŗČōŌŚŗćĪĀ”¢ŗćŃ¹Ģõ¼žĻĀĻņÉĻŹöĘ½ŗāĢåĻµÖŠĶØČėŗ¤Ęų£¬Ę½ŗā ŅĘ¶Æ£ØĢī”°Ļņ×ó”±”°ĻņÓŅ”±»ņ”°²»”±£©
¢ŚČōĪĀ¶ČĪŖT1”¢T2£¬·“Ó¦µÄĘ½ŗā³£Źż·Ö±šĪŖK1£¬K2£¬ŌņK1 K2£»·“Ó¦½ųŠŠµ½×“Ģ¬DŹ±£¬ 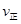
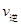 £ØĢī”°>”±”°<”±»ņ”°=”±£©
£ØĢī”°>”±”°<”±»ņ”°=”±£©
£Ø2£©µŖŹĒµŲĒņÉĻŗ¬Įæ·įø»µÄŅ»ÖÖŌŖĖŲ£¬µŖ¼°Ęä»ÆŗĻĪļŌŚ¹¤Å© ŅµÉś²ś”¢Éś»īÖŠÓŠ×ÅÖŲŅŖ×÷ÓĆ£¬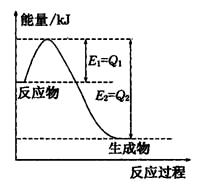
¢ŁČēĶ¼ŹĒŅ»¶ØµÄĪĀ¶ČŗĶŃ¹ĒæĻĀN2ŗĶH2·“Ӧɜ³ÉlmolNH3¹ż³ĢÖŠÄÜĮæ±ä»ÆŹ¾ŅāĶ¼£¬ĒėŠ“³ö¹¤ŅµŗĻ³É°±µÄČČ»Æѧ·½³ĢŹ½£ŗ
£Ø”÷HµÄŹżÖµÓĆŗ¬×ÖÄøQ1”¢Q2µÄ“śŹżŹ½±ķŹ¾£©
¢Ś°±ĘųČÜÓŚĖ®µĆµ½°±Ė®£¬ŌŚ25”ęĻĀ£¬½«a mol”¤L-1µÄ°±Ė®Óėb mol”¤L-1µÄŃĪĖįµČĢå»ż»ģŗĻ£¬·“Ó¦ŗóČÜŅŗĻŌÖŠŠŌ£¬Ōņc£ØNH4+£© c£ØCl-£©£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©£»ÓĆŗ¬aŗĶbµÄ“śŹżŹ½±ķŹ¾³öøĆ»ģŗĻČÜŅŗÖŠ°±Ė®µÄµēĄėĘ½ŗā³£Źż .
£Ø3£©ŗ£Ė®ÖŠŗ¬ÓŠ“óĮæµÄŌŖĖŲ£¬³£ĮæŌŖĖŲČēĀČ£¬Ī¢ĮæŌŖĖŲČēµā£¬ĘäŌŚŗ£Ė®ÖŠ¾łŅŌ»ÆŗĻĢ¬“ęŌŚ£¬ŌŚ25”ęĻĀ£¬Ļņ0£®1L0.002mol”¤L-lµÄNaClČÜŅŗÖŠÖšµĪ¼ÓČėŹŹĮæµÄ0£®1L0.002mol”¤L-lĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬“Ó³ĮµķČܽāĘ½ŗāµÄ½Ē¶Č½āŹĶ²śÉś³ĮµķµÄŌŅņŹĒ £¬Ļņ·“Ó¦ŗóµÄ×ĒŅŗÖŠ¼ĢŠų¼ÓČė0£®1L0.002mol”¤L-1µÄNaIČÜŅŗ£¬æ“µ½µÄĻÖĻóŹĒ £¬²śÉśøĆĻÖĻóµÄŌŅņŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£© ”£
£ØŅŃÖŖ£ŗ25”ꏱKSP£ØAgCl£©=1.6”Įl0-10 KSP£ØAgI£©=1.5”Įl0-16£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©”¢¢ŁÓĆėĀ(N2H4)ĪŖČ¼ĮĻ£¬ĖÄŃõ»Æ¶žµŖ×öŃõ»Æ¼Į£¬Į½Õß·“Ӧɜ³ÉµŖĘųŗĶĘųĢ¬Ė®”£
ŅŃÖŖ£ŗN2(g)£«2O2(g)£½N2O4(g) ¦¤H£½+10.7kJ”¤mol-1
N2H4(g)£«O2(g)£½N2(g)£«2H2O(g) ¦¤H£½-543kJ”¤mol-1
Š“³öĘųĢ¬ėĀŗĶN2O4·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
¢ŚŅŃÖŖĖÄŃõ»Æ¶žµŖŌŚ“óĘųÖŠ»ņŌŚ½ĻøßĪĀ¶ČĻĀŗÜÄŃĪČ¶Ø“ęŌŚ£¬ĘäŗÜČŻŅ××Ŗ»ÆĪŖ¶žŃõ»ÆµŖ”£ŹŌĶʶĻÓɶžŃõ»ÆµŖÖĘČ”ĖÄŃõ»Æ¶žµŖµÄ·“Ó¦Ģõ¼ž(»ņ“ėŹ©)£ŗ ”£
£Ø2£©æĘѧ¼ŅÖĘŌģ³öŅ»ÖÖŹ¹ÓĆ¹ĢĢåµē½āÖŹµÄČ¼ĮĻµē³Ų£¬Ę䊧ĀŹøüøߣ¬æÉÓĆÓŚŗ½Ģģŗ½æÕ”£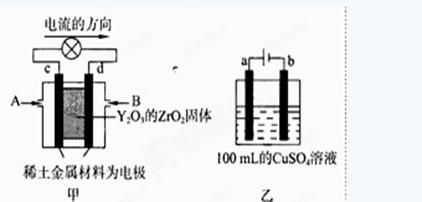
Ķ¼¼×ĖłŹ¾×°ÖĆÖŠ£¬ŅŌĻ”ĶĮ½šŹō²ÄĮĻĪŖ¶čŠŌµē¼«£¬ŌŚĮ½¼«ÉĻ·Ö±šĶØČėCH4ŗĶæÕĘų£¬ĘäÖŠ¹ĢĢåµē½āÖŹŹĒ²ōŌÓĮĖY2O3µÄZrO2¹ĢĢ壬ĖüŌŚøßĪĀĻĀÄÜ“«µ¼Ńō¼«Éś³ÉµÄO2-(O2+4e ”ś2O2-)
¢Łcµē¼«ĪŖ £¬dµē¼«ÉĻµÄµē¼«·“Ó¦Ź½ĪŖ ”£
¢ŚĶ¼ŅŅŹĒµē½ā100mL 0.5mol”¤L-1 CuSO4ČÜŅŗ£¬aµē¼«ÉĻµÄµē¼«·“Ó¦Ź½ĪŖ ”£Čōaµē¼«²śÉś56mL(±ź×¼×“æö)ĘųĢ壬ŌņĖłµĆČÜŅŗµÄpH= (²»æ¼ĀĒČÜŅŗĢå»ż±ä»Æ)£¬ČōŅŖŹ¹µē½āÖŹČÜŅŗ»Öø“µ½µē½āĒ°µÄדĢ¬£¬æɼÓČė (Ń”Ģī×ÖÄøŠņŗÅ)
a.CuO b.Cu(OH)2 c.CuCO3 d.Cu2(OH)2CO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŗĻ³É°±¹¤Ņµ¶Ō¹ś·Ą¾ßÓŠÖŲŅŖŅāŅ壬ČēÖĘĻõĖį”¢ŗĻ³ÉĻĖĪ¬ŅŌ¼°Č¾ĮĻµČ
£Ø1£©ŅŃÖŖijŠ©»Æѧ¼üµÄ¼üÄÜŹż¾ŻČēĻĀ±ķ£ŗ
| »Æѧ¼ü | N”ŌN | H”ŖH | N”ŖH |
| ¼üÄÜkJ”¤mol£1 | 946 | 436 | 390 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com