
ijŠ£»ÆѧŠĖȤŠ”×éÉč¼ĘŅŌĻĀ×°ÖĆ½ųŠŠ²»Ķ¬µÄŹµŃéĘäÖŠaĪŖÓĆÓŚ¹ÄČėæÕĘųµÄĘųÄŅ£¬bĪŖĀŻŠż×“ĶĖ棬CĪŖNaOHČÜŅŗ£¬dĪŖ±łĖ®»ģŗĻĪļ”£
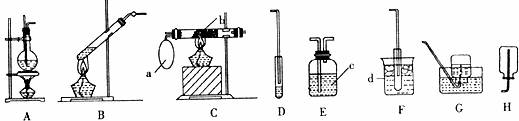
¢ń£®ÖĘČ”²¢ŹÕ¼ÆŅŅĻ©ĘųĢå£ŗøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £¬ŹµŃé¹ż³ĢÖŠ·¢ĻÖ·“Ó¦»ģŗĻĪļ±äŗŚ£¬¾Ż“ĖĶĘ²āÉś³ÉµÄŅŅĻ©ÖŠæÉÄÜŗ¬ÓŠµÄ¾ßÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢåŌÓÖŹŹĒ £¬øĆŹµŃéŃ”ÓƵÄ×°ÖĆŹĒ £ØĢī×°ÖƱąŗÅ£©”£
¢ņ£®ŅŅĖįŅŅõ„µÄÖĘČ”£ŗ”¢øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £»øĆŹµŃéŃ”ÓƵÄ×°ÖĆÓŠ £ØĢī×°ÖƱąŗÅ£©”£ŹµŃéæŖŹ¼Ź±£¬Ļņ·“ӦװÖĆÖŠĢķ¼ÓŹŌ¼ĮµÄĖ³ŠņĪŖ £¬Ļņ½ÓŹÕ×°ÖĆÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ £»ŹµŃé¹ż³ĢÖŠ£¬ĪŖ¼Óæģõ„»Æ·“Ó¦µÄĖŁĀŹ£¬Ķس£²ÉÓƵēėŹ©ŹĒ £»ŹµŃé½įŹųŹ±£¬ĪŖµĆµ½ŅŅĖįŅŅõ„¶ų½ųŠŠµÄ·ÖĄė²Ł×÷ŹĒ £ØŠ“³ö²Ł×÷Ćū³Ę£©”£
¢ó£® ŅŅ“¼µÄ“ß»ÆŃõ»ÆŹµŃé£ŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £¬ Ń”ÓƵÄ×°ÖĆŹĒ £ØĢī×°ÖƱąŗÅ£©”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
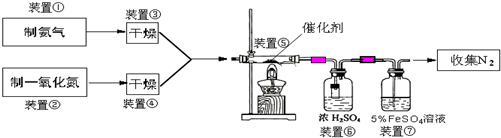
 £Ø2013?±£¶ØŅ»Ä££©Ä³Š£»ÆѧŠĖȤŠ”×éÉč¼ĘĮĖČēĻĀŹµŃé×°ÖĆ£ØĶ¼ÖŠ²æ·Ö¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£©Ą“²ā ¶ØijĢśĢ¼ŗĻ½šÖŠĢśµÄÖŹĮæ·ÖŹż£¬²¢Ģ½¾æĢśÓėÅØĮņĖįµÄ·“Ó¦£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø2013?±£¶ØŅ»Ä££©Ä³Š£»ÆѧŠĖȤŠ”×éÉč¼ĘĮĖČēĻĀŹµŃé×°ÖĆ£ØĶ¼ÖŠ²æ·Ö¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£©Ą“²ā ¶ØijĢśĢ¼ŗĻ½šÖŠĢśµÄÖŹĮæ·ÖŹż£¬²¢Ģ½¾æĢśÓėÅØĮņĖįµÄ·“Ó¦£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ| 3b |
| 11m |
| 3b |
| 11m |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
| m-c |
| m |
| m-c |
| m |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
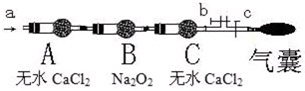
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«·šÉ½·šÉ½Ņ»ÖŠøßŅ»ĻĀѧʌµŚŅ»“Ī¶Īæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
ijŠ£»ÆѧŠĖȤŠ”×éÉč¼ĘĮĖĶ¼Ź¾ŹµŃé×°ÖĆ£ØĶ¼ÖŠŹ”ĀŌĮĖ¼Š³ÖŅĒĘ÷£©Ą“²ā¶ØijĢśĢ¼ŗĻ½šÖŠĢśµÄÖŹĮæ·ÖŹż”£
£Ø1£©m gĢśĢ¼ŗĻ½šÖŠ¼ÓČė¹żĮæÅØĮņĖį£¬Ī“µćČ¼¾Ę¾«µĘĒ°£¬A”¢B¾łĪŽĆ÷ĻŌĻÖĻó£¬ĘäŌŅņŹĒ£ŗ¢Ł³£ĪĀĻĀĢ¼ÓėÅØĮņĖį²»·“Ó¦£»¢Ś_____________”£
£Ø2£©Š“³ö¼ÓČČŹ±AÖŠĢ¼ÓėÅØĮņĖį·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½____________”£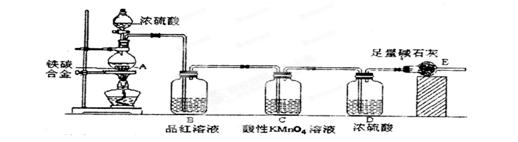
£Ø3£©BÖŠµÄĻÖĻóŹĒ£ŗ____________£»CµÄ×÷ÓĆŹĒ£ŗ_______________”£
£Ø4£©“żAÖŠ²»ŌŁŅŻ³öĘųĢåŹ±£¬Ķ£Ö¹¼ÓČČ£¬²šĻĀE²¢³ĘÖŲ£¬EŌöÖŲb g”£ŌņĢśĢ¼ŗĻ½šÖŠĢśµÄÖŹĮæ·ÖŹżĪŖ_____________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com