
ΓΨΧβΡΩΓΩΘ®1Θ©œ¬Ν–Ζ¥”ΠΩ…”Ο”ΎΦλ≤βΥΨΜζ «ΖώΨΤΚσΦί ΜΘΚ![]()
![]() ΓΘ
ΓΘ
ΔΌ≈δΚœΈο![]() ÷–Θ§”κ
÷–Θ§”κ![]() –Έ≥…≈δΈΜΦϋΒΡ‘≠Ή” «__________Θ®Χν‘ΣΥΊΖϊΚ≈Θ©ΓΘ
–Έ≥…≈δΈΜΦϋΒΡ‘≠Ή” «__________Θ®Χν‘ΣΥΊΖϊΚ≈Θ©ΓΘ
ΔΎ![]() ÷–
÷–![]() ‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά «________ΘΜ
‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά «________ΘΜ![]()
![]() Κ§”–
Κ§”–![]() ΦϋΒΡ ΐΡΩΈΣ_______ΓΘ
ΦϋΒΡ ΐΡΩΈΣ_______ΓΘ
Θ®2Θ©![]() Ζ÷Ή”÷–Θ§
Ζ÷Ή”÷–Θ§![]() ‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά «__________ΘΜ–¥≥ω”…3Ηω‘≠Ή”Ήι≥…«“”κ
‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά «__________ΘΜ–¥≥ω”…3Ηω‘≠Ή”Ήι≥…«“”κ![]() ΨΏ”–œύΆ§Ω’ΦδΙΙ–ΆΒΡάκΉ”ΘΚ___________Θ®Χν“ΜΗωΦ¥Ω…Θ©ΓΘ
ΨΏ”–œύΆ§Ω’ΦδΙΙ–ΆΒΡάκΉ”ΘΚ___________Θ®Χν“ΜΗωΦ¥Ω…Θ©ΓΘ
Θ®3Θ© ·ΡΪœ©Θ®ΫαΙΙ»γΆΦ1Υυ ΨΘ© «“Μ÷÷”…ΒΞ≤ψΧΦ‘≠Ή”ΙΙ≥…ΒΡΨΏ”–ΤΫΟφΫαΙΙΒΡ–¬–ΆΧΦ≤ΡΝœΘ§ ·ΡΪœ©÷–≤ΩΖ÷ΧΦ‘≠Ή”±Μ―θΜ·ΚσΘ§ΤδΤΫΟφΫαΙΙΜαΖΔ…ζΗΡ±δΘ§ΉΣΜ·ΈΣ―θΜ· ·ΡΪœ©Θ®ΫαΙΙ»γΆΦ2Υυ ΨΘ©ΓΘ

―θΜ· ·ΡΪœ©÷–2Κ≈![]() ‘≠Ή”ΒΡ‘”Μ·ΖΫ Ϋ «_________Θ§ΗΟ
‘≠Ή”ΒΡ‘”Μ·ΖΫ Ϋ «_________Θ§ΗΟ![]() ‘≠Ή””κœύΝΎ
‘≠Ή””κœύΝΎ![]() ‘≠Ή”–Έ≥…ΒΡΦϋΫ«______Θ®ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±Θ© ·ΡΪœ©÷–1Κ≈
‘≠Ή”–Έ≥…ΒΡΦϋΫ«______Θ®ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±Θ© ·ΡΪœ©÷–1Κ≈![]() ”κœύΝΎ
”κœύΝΎ![]() –Έ≥…ΒΡΦϋΫ«ΓΘ
–Έ≥…ΒΡΦϋΫ«ΓΘ
ΓΨ¥πΑΗΓΩ![]()
![]() ‘”Μ·ΓΔ
‘”Μ·ΓΔ![]() ‘”Μ·
‘”Μ· ![]()
![]() ‘”Μ·
‘”Μ· ![]() Θ®Μρ
Θ®Μρ![]() Β»Θ©
Β»Θ© ![]() ‘”Μ· <
‘”Μ· <
ΓΨΫβΈωΓΩ
Θ®1Θ©ΔΌ≈δΚœΈο![]() ÷–
÷–![]() ΈΣ÷––ΡάκΉ”Θ§
ΈΣ÷––ΡάκΉ”Θ§![]() ΈΣ≈δΧεΘ§
ΈΣ≈δΧεΘ§![]() ‘≠Ή”ΧαΙ©Ι¬Ε‘ΒγΉ”Θ§”κ
‘≠Ή”ΧαΙ©Ι¬Ε‘ΒγΉ”Θ§”κ![]() –Έ≥…≈δΈΜΦϋΘ§Ι ¥πΑΗΈΣΘΚ
–Έ≥…≈δΈΜΦϋΘ§Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
ΔΎ![]() ÷–
÷–![]() ‘≠Ή”Ζ÷±π–Έ≥…4Ηω
‘≠Ή”Ζ÷±π–Έ≥…4Ηω![]() ΦϋΓΔ3Ηω
ΦϋΓΔ3Ηω![]() ΦϋΘ§ΨυΟΜ”–Ι¬Ε‘ΒγΉ”Θ§Ζ÷±πΈΣ
ΦϋΘ§ΨυΟΜ”–Ι¬Ε‘ΒγΉ”Θ§Ζ÷±πΈΣ![]() ‘”Μ·ΓΔ
‘”Μ·ΓΔ![]() ‘”Μ·ΘΜΗυΨί
‘”Μ·ΘΜΗυΨί![]() ΒΡΫαΙΙ ΫΩ…÷ΣΘ§
ΒΡΫαΙΙ ΫΩ…÷ΣΘ§![]() Ζ÷Ή”÷–Κ§”–
Ζ÷Ή”÷–Κ§”–![]() ΦϋΒΡ ΐΡΩΈΣ
ΦϋΒΡ ΐΡΩΈΣ![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ‘”Μ·ΓΔ
‘”Μ·ΓΔ![]() ‘”Μ·ΘΜ
‘”Μ·ΘΜ![]() ΘΜ
ΘΜ
Θ®2Θ©”…3Ηω‘≠Ή”Ήι≥…«“”κ![]() ΨΏ”–œύΆ§Ω’ΦδΙΙ–ΆΒΡάκΉ””κ
ΨΏ”–œύΆ§Ω’ΦδΙΙ–ΆΒΡάκΉ””κ![]() ΜΞΈΣΒ»ΒγΉ”ΧεΘ§≥ΘΦϊΒΡ”–
ΜΞΈΣΒ»ΒγΉ”ΧεΘ§≥ΘΦϊΒΡ”–![]() ΓΔ
ΓΔ![]() Β»Θ§Ι ¥πΑΗΈΣΘΚ
Β»Θ§Ι ¥πΑΗΈΣΘΚ![]() ‘”Μ·ΘΜ
‘”Μ·ΘΜ![]() Θ®Μρ
Θ®Μρ![]() Β»Θ©ΘΜ
Β»Θ©ΘΜ
Θ®3Θ©―θΜ· ·ΡΪœ©÷–2Κ≈![]() ‘≠Ή”–Έ≥…3Ηω
‘≠Ή”–Έ≥…3Ηω![]() ΦϋΚΆ1Ηω
ΦϋΚΆ1Ηω![]() ΦϋΘ§
ΦϋΘ§![]() ‘≠Ή”≤…»Γ
‘≠Ή”≤…»Γ![]() ‘”Μ·Θ§ΗΟ
‘”Μ·Θ§ΗΟ![]() ‘≠Ή”ΚΆ”κΤδœύΝ§ΒΡ4Ηω‘≠Ή”–Έ≥…ΥΡΟφΧεΘ§Εχ ·ΡΪœ©÷–ΒΡ1Κ≈
‘≠Ή”ΚΆ”κΤδœύΝ§ΒΡ4Ηω‘≠Ή”–Έ≥…ΥΡΟφΧεΘ§Εχ ·ΡΪœ©÷–ΒΡ1Κ≈![]() ‘≠Ή”–Έ≥…3Ηω
‘≠Ή”–Έ≥…3Ηω![]() ΦϋΘ§ΆΦ1ΈΣΤΫΟφΫαΙΙΘ§
ΦϋΘ§ΆΦ1ΈΣΤΫΟφΫαΙΙΘ§![]() ‘≠Ή”≤…»Γ
‘≠Ή”≤…»Γ![]() ‘”Μ·Θ§ΗΟ
‘”Μ·Θ§ΗΟ![]() ‘≠Ή”ΚΆ”κΤδœύΝ§ΒΡ3Ηω‘≠Ή”–Έ≥…ΤΫΟφ»ΐΫ«–ΈΘ§‘ρ―θΜ· ·ΡΪœ©÷–2Κ≈
‘≠Ή”ΚΆ”κΤδœύΝ§ΒΡ3Ηω‘≠Ή”–Έ≥…ΤΫΟφ»ΐΫ«–ΈΘ§‘ρ―θΜ· ·ΡΪœ©÷–2Κ≈![]() ‘≠Ή””κœύΝΎ
‘≠Ή””κœύΝΎ![]() ‘≠Ή”–Έ≥…ΒΡΦϋΫ«< ·ΡΪœ©÷–1Κ≈
‘≠Ή”–Έ≥…ΒΡΦϋΫ«< ·ΡΪœ©÷–1Κ≈![]() ‘≠Ή””κœύΝΎ
‘≠Ή””κœύΝΎ![]() ‘≠Ή”–Έ≥…ΒΡΦϋΫ«Θ§Ι ¥πΑΗΈΣΘΚ
‘≠Ή”–Έ≥…ΒΡΦϋΫ«Θ§Ι ¥πΑΗΈΣΘΚ![]() ‘”Μ·ΘΜ<ΓΘ
‘”Μ·ΘΜ<ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩCO «¥σΤχΈέ»ΨΤχΧεΘ§Ω…άϊ”ΟΜ·―ßΖ¥”ΠΫχ––÷ΈάμΜρΉΣΜ·ΓΘ
(1)ΦΉ¥Φ «÷Ί“ΣΒΡ»ήΦΝΚΆ»ΦΝœΘ§ΙΛ“Β…œ”ΟCOΚΆH2‘Ύ“ΜΕ®ΧθΦΰœ¬÷Τ±ΗCH3OHΒΡΖ¥”ΠΈΣΘΚCO(g)ΘΪ2H2(g)![]() CH3OH(g) ΓςH<0
CH3OH(g) ΓςH<0
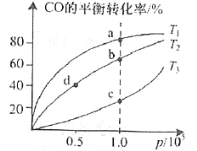
ΔΌTΓφ ±Θ§œρ»ίΜΐΈΣ2LΒΡΚψ»ίΟή±’»ίΤς÷–≥δ»κ1 mol COΚΆ1.2 mol H2Θ§“ΜΕΈ ±ΦδΚσ¥οΒΫΤΫΚβΘ§¥Υ ±H2”κCH3OHΒΡΧεΜΐΖ÷ ΐ÷°±»ΈΣ2ΘΚ5Θ§ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKΘΫ___________ΘΜ¥Υ ±»τœρ»ίΤς÷–‘ΌΆ®»κ0.4 mol COΚΆ0.2 mol CH3OH(g)Θ§‘ρΤΫΚβΫΪ___________“ΤΕ·ΓΘ(ΧνΓΑœρ’ΐΖ¥”ΠΖΫœρΓ±ΓΑ≤ΜΓ±ΜρΓΑœρΡφΖ¥”ΠΖΫœρΓ±)
ΔΎ‘Ύ“Μ»ίΜΐΩ…±δΒΡΟή±’»ίΤς÷–≥δ»κ“ΜΕ®Έο÷ ΒΡΝΩΒΡCOΚΆH2Θ§≤βΒΟCO‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡΤΫΚβΉΣΜ·¬ ”κ―Ι«ΩΒΡΙΊœΒ»γΆΦΥυ ΨΓΘaΓΔbΓΔc»ΐΒψΤΫΚβ≥Θ ΐK(a)ΓΔK(b)ΓΔK(c)ΒΡ¥σ–ΓΙΊœΒ «___________ΓΘbΓΔdΒψΒΡ’ΐΖ¥”ΠΥΌ¬ vb(CO)_______va(CO).
(2)ΝΛ«ύΜλΡΐΆΝΩ…ΉςΈΣ2CO(g)ΘΪO2(g)![]() 2CO2(g)Ζ¥”ΠΒΡ¥ΏΜ·ΦΝΓΘΆΦ±μ Ψ‘ΎœύΆ§ΒΡΚψ»ίΟή±’»ίΤςΓΔœύΆ§Τπ Φ≈®Ε»ΓΔΖ¥”ΠœύΆ§ΒΡ ±ΦδΘ§ Ι”ΟΆ§÷ ΝΩΒΡ≤ΜΆ§ΝΛ«ύΜλΡΐΆΝ(ΠΝ–ΆΓΔΠ¬–Ά)¥ΏΜ· ±Θ§COΒΡΉΣΜ·¬ ”κΈ¬Ε»ΒΡΙΊœΒΓΘ
2CO2(g)Ζ¥”ΠΒΡ¥ΏΜ·ΦΝΓΘΆΦ±μ Ψ‘ΎœύΆ§ΒΡΚψ»ίΟή±’»ίΤςΓΔœύΆ§Τπ Φ≈®Ε»ΓΔΖ¥”ΠœύΆ§ΒΡ ±ΦδΘ§ Ι”ΟΆ§÷ ΝΩΒΡ≤ΜΆ§ΝΛ«ύΜλΡΐΆΝ(ΠΝ–ΆΓΔΠ¬–Ά)¥ΏΜ· ±Θ§COΒΡΉΣΜ·¬ ”κΈ¬Ε»ΒΡΙΊœΒΓΘ
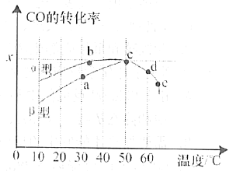
ΔΌaΓΔbΓΔcΓΔdΥΡΒψ÷–±μ ΨΤΫΚβΉ¥Χ§ΒΡ «___________ΘΜ
ΔΎeΒψΉΣΜ·¬ ≥ωœ÷ΆΜ±δΒΡ‘≠“ρΩ…Ρή «______________________ΓΘ
(3)ΒγΫβΖ®ΉΣΜ·CO2Ω… Βœ÷CO2Ή ‘¥Μ·άϊ”ΟΓΘΒγΫβ ±CO2‘Ύ“θΦΪ«χΉΣΜ·ΈΣHCOOHΘ§Τδ‘≠άμ Ψ“βΆΦ»γœ¬ΘΚ
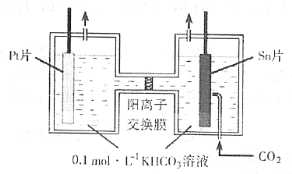
ΒγΫβ“ΜΕΈ ±ΦδΚσΘ§―τΦΪ«χΒΡKHCO3»ή“Κ≈®Ε»ΫΒΒΆΘ§Τδ‘≠“ρ «_________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ1Θ§2-Ε଻±ϊΆι(CH2ClCHClCH3) «“Μ÷÷÷Ί“ΣΒΡΜ·ΙΛ‘≠ΝœΘ§ΙΛ“Β…œΩ…”Ο±ϊœ©Φ”≥…Ζ®÷Τ±ΗΘ§÷ς“ΣΗ±≤ζΈοΈΣ3-¬»±ϊœ©(CH2=CHCH2Cl)Θ§Ζ¥”Π‘≠άμΈΣΘΚ
IΘ°CH2=CHCH3(g)+Cl2(g) ![]() CH2ClCHClCH3(g) H1=-134kJΓΛmol-1
CH2ClCHClCH3(g) H1=-134kJΓΛmol-1
IIΘ°CH2=CHCH3(g)+Cl2(g) CH2=CHCH2Cl(g)+HCl(g) H2=-102kJΓΛmol-1
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)“―÷ΣCH2=CHCH2Cl(g)+HCl(g) ![]() CH2ClCHClCH3(g)ΒΡΜνΜ·ΡήEa(’ΐ)ΈΣ132kJΓΛmol-1Θ§‘ρΗΟΖ¥”ΠΒΡΜνΜ·ΡήEa(Ρφ)ΈΣ___________kJΓΛmol-1ΓΘ
CH2ClCHClCH3(g)ΒΡΜνΜ·ΡήEa(’ΐ)ΈΣ132kJΓΛmol-1Θ§‘ρΗΟΖ¥”ΠΒΡΜνΜ·ΡήEa(Ρφ)ΈΣ___________kJΓΛmol-1ΓΘ
(2)“ΜΕ®Έ¬Ε»œ¬Θ§Οή±’»ίΤς÷–ΖΔ…ζΖ¥”ΠIΚΆΖ¥”ΠIIΘ§¥οΒΫΤΫΚβΚσ‘ω¥σ―Ι«ΩΘ§CH2ClCHClCH3ΒΡ≤ζ¬ ____________Θ®ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±Θ©Θ§άμ”… «_________________________________ΓΘ
(3)Τπ Φ ±œρΡ≥Κψ»ίΨχ»»»ίΤς÷–≥δ»κ1 mol CH2=CHCH3ΚΆ1 mol Cl2ΖΔ…ζΖ¥”ΠIIΘ§¥οΒΫΤΫΚβ ±Θ§»ίΤςΡΎΤχΧε―Ι«Ω_________________Θ®ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±Θ©ΓΘ
(4)Ρ≥―–ΨΩ–ΓΉιœρΟή±’»ίΤς÷–≥δ»κ“ΜΕ®ΝΩΒΡCH2=CHCH3ΚΆCl2Θ§Ζ÷±π‘ΎAΓΔBΝΫ÷÷≤ΜΆ§¥ΏΜ·ΦΝΉς”Οœ¬ΖΔ…ζΖ¥”ΠΘ§“ΜΕΈ ±ΦδΚσ≤βΒΟCH2ClCHClCH3ΒΡ≤ζ¬ ”κΈ¬Ε»ΒΡΙΊœΒ»γΆΦΥυ ΨΓΘpΒψ «ΖώΈΣΕ‘”ΠΈ¬Ε»œ¬CH2ClCHClCH3ΒΡΤΫΚβ≤ζ¬ Θ§_________ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©≈–Εœάμ”… «_______________________ΓΘ

(5)“ΜΕ®Έ¬Ε»œ¬Θ§œρΚψ»ίΟή±’»ίΤς÷–≥δ»κΒ»Έο÷ ΒΡΝΩΒΡCH2=CHCH3(g)ΚΆCl2(g)ΓΘ‘Ύ¥ΏΜ·ΦΝΉς”Οœ¬ΖΔ…ζΖ¥”ΠIΘ§»ίΤςΡΎΤχΧεΒΡ―Ι«ΩΥφ ±ΦδΒΡ±δΜ·»γœ¬±μΥυ ΨΓΘ
±Φδ/min | 0 | 60 | 120 | 180 | 240 | 300 | 360 |
―Ι«Ω/kPa | 80 | 74.2 | 69.4 | 65.2 | 61.6 | 57.6 | 57.6 |
ΔΌ”ΟΒΞΈΜ ±ΦδΡΎΤχΧεΖ÷―ΙΒΡ±δΜ·ά¥±μ ΨΖ¥”ΠΥΌ¬ Θ§Φ¥![]()
ΔΎΗΟΈ¬Ε»œ¬Θ§»τΤΫΚβ ±HClΒΡΧεΜΐΖ÷ ΐΈΣ![]() Θ§‘ρ±ϊœ©ΒΡΤΫΚβΉήΉΣΜ·¬
Θ§‘ρ±ϊœ©ΒΡΤΫΚβΉήΉΣΜ·¬ ![]() ____________ΘΜΖ¥”ΠIΒΡΤΫΚβ≥Θ ΐKp=____________________kPa-1(KpΈΣ“‘Ζ÷―Ι±μ ΨΒΡΤΫΚβ≥Θ ΐΘ§±ΘΝτ–Γ ΐΒψΚσ2ΈΜ)ΓΘ
____________ΘΜΖ¥”ΠIΒΡΤΫΚβ≥Θ ΐKp=____________________kPa-1(KpΈΣ“‘Ζ÷―Ι±μ ΨΒΡΤΫΚβ≥Θ ΐΘ§±ΘΝτ–Γ ΐΒψΚσ2ΈΜ)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ»γΆΦ «Β¬ΙζΜ·―ßΦ“άν±»œΘ1831Ρξ≤βΕ®ΧΰάύΜ·ΚœΈοΘ®÷ΜΚ§CΓΔHΝΫ÷÷‘ΣΥΊΘ©Ήι≥…ΒΡΉΑ÷ΟΓΘ¥…÷έΡΎΒΡΧΰ―υΤΖΨ≠Φ”»»Ζ÷ΫβΜρΤχΜ·Κσ”Ο¥Ω![]() Νς«ΐΗœΨ≠ΙΐΉΤ»»ΒΡ
Νς«ΐΗœΨ≠ΙΐΉΤ»»ΒΡ![]() Θ§’βάοΧΰάύΜ·ΚœΈοΖ¥”Π±δ≥…
Θ§’βάοΧΰάύΜ·ΚœΈοΖ¥”Π±δ≥…![]() ΚΆ
ΚΆ![]() Θ§Ψ≠Έϋ ’ΙήΈϋ ’ΓΘ
Θ§Ψ≠Έϋ ’ΙήΈϋ ’ΓΘ

“―÷Σœ¬±μ ΐΨίΘΚ
¥…÷έ | Έϋ ’ΙήΔώ | Έϋ ’ΙήΔρ | |||
¥…÷έ | ―υΤΖ+¥…÷έ | Έϋ ’«Α | Έϋ ’Κσ | Έϋ ’«Α | Έϋ ’Κσ |
A | B | C | D | E | F |
(1)Χΰ―υΤΖ‘ΎΖ¥”ΠΙΐ≥Χ÷–Υυ”ΟΒΡ―θΜ·ΦΝ «________________________ΘΜ
(2)Έϋ ’ΙήΔώ”ΠΉΑΒΡΈϋ ’ΦΝ «____________Θ§Έϋ ’ΙήΔρ”ΠΉΑΒΡΈϋ ’ΦΝ «____________ΘΜ
(3)―υΤΖ÷–ΧΦΒΡ÷ ΝΩΖ÷ ΐΒΡ ΐ―ß±μ¥ο Ϋ «________________________ΘΜ
(4)»ΓΗΟΧΰ![]() ‘Ύ―θΤχ÷–≥δΖ÷»Φ…’ΚσΘ§…ζ≥…
‘Ύ―θΤχ÷–≥δΖ÷»Φ…’ΚσΘ§…ζ≥…![]() ΚΆ
ΚΆ![]() ΓΘ»τΗΟΧΰ‘Ύ“ΜΕ®ΧθΦΰœ¬ΡޔꬻΤχΖΔ…ζ»Γ¥ζΖ¥”ΠΘ§«“Τδ“Μ¬»¥ζΈο÷Μ”–“Μ÷÷Θ§‘ρΧΰAΒΡΫαΙΙΦρ ΫΈΣ___________Θ§”ΟœΒΆ≥ΟϋΟϊΖ®ΟϋΟϊΈΣ____________ΓΘ
ΓΘ»τΗΟΧΰ‘Ύ“ΜΕ®ΧθΦΰœ¬ΡޔꬻΤχΖΔ…ζ»Γ¥ζΖ¥”ΠΘ§«“Τδ“Μ¬»¥ζΈο÷Μ”–“Μ÷÷Θ§‘ρΧΰAΒΡΫαΙΙΦρ ΫΈΣ___________Θ§”ΟœΒΆ≥ΟϋΟϊΖ®ΟϋΟϊΈΣ____________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ±μ÷– «‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ§ΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
Ήε ÷ήΤΎ | ΔώA | ΔρA | ΔσA | ΔτA | ΔθA | ΔωA | ΔςA | 0 |
2 | ΔΌ | ΔΎ | ||||||
3 | Δέ | Δή | Δί | Δό | ΔΏ | Δύ | Δα | |
4 | Δβ |
(1)–¥≥ωœ¬Ν–‘ΣΥΊΖϊΚ≈ΘΚΔΌ_______Θ§Δό_______Θ§ΔΏ_______Θ§_________ΓΘ
(2)‘Ύ’β–©‘ΣΥΊ–Έ≥…ΒΡΒΞ÷ ÷–Θ§ΉνΜνΤΟΒΡΫπ τΒΞ÷ «_____________Θ§ΉνΜνΤΟΒΡΖ«Ϋπ τΒΞ÷ «_________Θ®”ΟΜ·―ßΖϊΚ≈Ήω¥πΘ©ΓΘ
(3)‘Ύ’β–©‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·Έο÷–Θ§Υα–‘Ήν«ΩΒΡ «Θ®ΧνΜ·―ß ΫΘ§œ¬Ά§Θ©____________Θ§Φν–‘Ήν«ΩΒΡ «____________Θ§≥ ΝΫ–‘ΒΡ «___________Θ§“‘…œ»ΐ’Ώ÷°ΦδœύΜΞΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ________________________ΘΜ________________________ΘΜ________________________
(4)‘ΎΔή”κΔί–Έ≥…ΒΡΒΞ÷ ÷–Θ§Μ·―ß–‘÷ ΫœΜνΤΟΒΡ «____________Θ®ΧνΟϊ≥ΤΘ©Θ§…ηΦΤΝΫΗω‘≠άμ≤ΜΆ§ΒΡΦρΒΞ Β―ιΓΘ
ΖΫΑΗ“ΜΘΚ________________________________________________ΘΜ
ΖΫΑΗΕΰΘΚ________________________________________________ΓΘ
(5)‘ΎΔΏ”κΔύ–Έ≥…ΒΡΒΞ÷ ÷–Θ§Μ·―ß–‘÷ ΫœΜνΤΟΒΡ «____________Θ§–¥≥ωΩ…“‘÷ΛΟςΗΟΫα¬έΒΡ“ΜΗωάκΉ”Ζ¥”ΠΖΫ≥Χ Ϋ________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–”κΫπ τΗ· ¥”–ΙΊΒΡΥΒΖ®Θ§’ΐ»ΖΒΡ «Θ® Θ©
A.ΆΦ1÷–Θ§≤ε»κΚΘΥ°÷–ΒΡΧζΑτΘ§‘ΫΩΩΫϋΒΉΕΥΗ· ¥‘Ϋ―œ÷Ί
B.ΆΦ2÷–Θ§≤ε»κ»ή“Κ÷–ΒΡΧζΑτ»ί“Ή»ήΫβΘ§÷ς“Σ «ΖΔ…ζΒγΜ·―ßΗ· ¥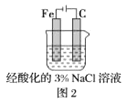
C.ΆΦ3÷–Θ§»ΦΤχ‘νΒΡ÷––Ρ≤ΩΈΜ»ί“Ή…ζ–βΘ§÷ς“Σ «”…”ΎΗΏΈ¬œ¬ΧζΖΔ…ζΒγΜ·―ßΗ· ¥
D.ΆΦ4÷–Θ§”ΟΈΰ…ϋΟΨΩιΒΡΖΫΖ®ά¥Ζά÷ΙΒΊœ¬Η÷ΧζΙήΒάΒΡΗ· ¥Θ§ΟΨΩιœύΒ±”Ύ‘≠Βγ≥ΊΒΡΗΚΦΪ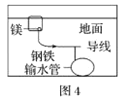
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν– ¬ Β≤ΜΡή÷ΛΟςHNO2 «»θΒγΫβ÷ ΒΡ «Θ® Θ©
ΔΌΒΈ»κΖ”ΧΣΘ§NaNO2»ή“Κœ‘Κλ…ΪΔΎ”ΟHNO2»ή“ΚΉωΒΦΒγ Β―ιΘ§ΒΤ≈ίΚήΑΒ
ΔέΒ»pHΓΔΒ»ΧεΜΐΒΡ―ΈΥαΚΆHNO2»ή“Κ÷–ΚΆΦν ±Θ§HNO2ΒΡ÷–ΚΆΦνΡήΝΠ«Ω
Δή0.1molL-1HNO2»ή“ΚΒΡpH=2ΔίHNO2”κCaCO3Ζ¥”ΠΖ≈≥ωCO2ΤχΧε
Δόc(HΘΪ)=0.1molL-1ΒΡHNO3»ή“ΚœΓ Ά÷Ν1000±ΕΘ§pHΘΦ4
A.ΔΎΔίB.ΔΌΔίC.ΔέΔόD.ΔέΔή
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΒΣΜ·ΗθΨΏ”–ΦΪΗΏΒΡ”≤Ε»ΚΆΝΠ―ß«ΩΕ»ΓΔ”≈“λΒΡΩΙΗ· ¥–‘ΡήΚΆΗΏΈ¬Έ»Ε®–‘ΡήΘ§“ρΕχΨΏ”–ΙψΖΚ”Π”Ο«ΑΨΑΓΘ Β―ι “÷Τ±ΗCrN Ζ¥”Π‘≠άμΈΣ CrCl3 +NH3![]() CrN+3HClΘ§ΉΑ÷Ο»γΆΦΥυ Ψ
CrN+3HClΘ§ΉΑ÷Ο»γΆΦΥυ Ψ
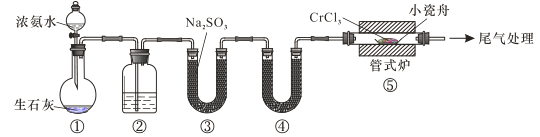
œ¬Ν–ΥΒΖ®¥μΈσΒΡ «
A.ΉΑ÷ΟΔΌΜΙΩ…“‘”Ο”Ύ÷Τ»ΓO2ΓΔCl2ΓΔCO2Β»ΤχΧε
B.ΉΑ÷ΟΔΎΓΔΔή÷–Ζ÷±π ΔΉΑNaOH»ή“ΚΓΔKOHΙΧΧε
C.ΉΑ÷ΟΔέ÷–“≤Ω… ΔΉΑΈ§…ζΥΊcΘ§ΤδΉς”Ο «≥ΐ»Ξ―θΤχ
D.ΉΑ÷ΟΔί ÷–≤ζ…ζΒΡΈ≤Τχά以Κσ”ΟΥ°Έϋ ’ΒΟΒΫ¥Ω―ΈΥα
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΆ≠ΚΆΝρΒΡΜ·ΚœΈο‘ΎΜ·ΙΛΓΔ“Ϋ“©≤ΡΝœΒ»Νλ”ρΨΏ”–ΙψΖΚΒΡ”ΟΆΨΓΘΜΊ¥πœ¬Ν–Έ Χβ:
Θ®1Θ©‘≠Ή”ΙλΒά «÷ΗΒγΉ”‘Ύ‘≠Ή”ΚΥΆβΒΡ___________Θ§ΜυΧ§S‘≠Ή”ΒΡ‘≠Ή”ΙλΒά ΐ «____ΗωΓΘ
Θ®2Θ©ΜυΧ§Cu‘≠Ή”÷–,ΚΥΆβΒγΉ”’ΦΨίΒΡ‘≠Ή”ΙλΒάΈΣ«ρ–ΈΒΡΉνΗΏΡήΦΕΖϊΚ≈ «______Θ§’ΦΨίΗΟΡήΦΕΒΡΒγΉ” ΐΈΣ__________ΓΘ
Θ®3Θ©ClΓΔSΓΔSe‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–¥Π”ΎœύΝΎΒΡΈΜ÷Ο,ΤδΒΎ“ΜΒγάκΡήΒΡ¥σ–ΓΥ≥–ρΈΣ_______ΓΘ
Θ®4Θ©œ¬ΆΦ «Κ§‘ΣΥΊCuΓΔSΒΡ”–ΜζΈοΒΡΫαΙΙΦρ Ϋ:

ΔΌΗΟ”–ΜζΜ·ΚœΈοΫαΙΙ÷–Κ§”–ΒΡΜ·―ßΦϋάύ–Ά «_______(ΧνΓΑΙ≤ΦέΦϋΓΑΓΑάκΉ”ΦϋΓ±ΜρΓΑΙ≤ΦέΦϋΓΔάκΉ”ΦϋΓ±)ΓΔ≈δΈΜΦϋ,Τδ÷–1ΗωΗΟ”–ΜζΈοΖ÷Ή”÷–≈δΈΜΦϋ ΐΈΣ_____Ηω,’β–©≈δΈΜΦϋ÷–ΧαΙ©Ι¬ΒγΉ”Ε‘ΒΡ‘ΣΥΊ «__________ΓΘ
ΔΎS‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ_______ΓΔ¥χ*N‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ_______ΓΘ
Θ®5Θ©œ¬ΆΦ «Cu-AuΚœΫπΒΡ“Μ÷÷ΝΔΖΫΨßΧεΫαΙΙ:

“―÷ΣΗΟΚœΫπΒΡΟήΕ»Ζ÷dg/cm3,ΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΈΣNAΘ§»τAu‘≠Ή”ΒΡΑκΨΕΈΣbpm(lpm=10-10cm)Θ§‘ρΆ≠‘≠Ή”ΒΡΑκΨΕΈΣ______cm(–¥≥ωΦΤΥψ±μ¥ο Ϋ)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com