
ĪŖĮĖŌ¤·Ą»·¾³ĪŪČ¾²¢¶ŌĪ²Ęų½ųŠŠ×ŪŗĻĄūÓĆ£¬Ä³ĮņĖį³§ÓĆ°±Ė®ĪüŹÕĪ²ĘųÖŠµÄSO2£®ŌŁĻņĪüŹÕŅŗÖŠ¼ÓČėÅØĮņĖį£¬ŅŌÖĘČ”øßÅØ¶ČµÄSO2¼°(NH4)2SO4ŗĶNH4HSO4¹ĢĢ壮
ĪŖ²ā¶ØÉĻŹö(NH4)2SO4ŗĶNH4HSO4¹ĢĢå»ģŗĻĪļµÄ×é³É£¬ĻÖ³ĘČ”øĆѳʷ4·Ż£¬·Ö±š¼ÓČėĻąĶ¬ÅØ¶ČµÄNaOHČÜŅŗø÷40.00 mL£¬¼ÓČČÖĮ120”ę×óÓŅ£¬Ź¹°±Č«²æŅŻ³ö[(NH4)2SO4ŗĶNH4HSO4¹ĢĢå·Ö½āµÄĪĀ¶Č¾łøßÓŚ200”ę]£¬²āµĆÓŠ¹ŲŹµŃ鏿¾ŻČēĻĀ(±ź×¼×“æöĻĀ)£ŗ
(1)ŹµŃé¹ż³ĢÖŠÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________£®
(2)ÓÉ¢ń×鏿¾ŻÖ±½ÓŌ¤²ā£ŗ±ź×¼×“æöĻĀ3.7 gѳʷ½ųŠŠĶ¬ŃłŹµŃ鏱£¬Éś³É°±µÄĢå»żĪŖ________L£®
(3)øĆ»ģŗĻĪļÖŠ(NH4)2SO4ŗĶNH4HSO4µÄĪļÖŹµÄĮæÖ®±ČĪŖ________£®
|
””””(1)(NH4)2SO4ŗĶNH4HSO4ŌŚČÜŅŗÖŠŹĒĶźČ«µēĄėµÄ£¬ĖłŅŌ(NH4)2SO4ŗĶNH4HSO4¹ĢĢå»ģŗĻĪļÖŠ¼ÓČėNaOHČÜŅŗŹ±£¬½«ÓŠŅŌĻĀĮ½øöĄė×Ó·“Ó¦·¢Éś£ŗ ””””H+£«OH££½H2O£¬ ””””(2)ÓɱķÖŠŹż¾ŻæÉ擳ö£¬µ±ŃłĘ·ÖŹĮæÓÉ7.4 gŌö¼Óµ½14.8 gŹ±£¬ŹĶ·Å³öµÄĘųĢåĢå»żÓÉ1.68 LŌö¼Óµ½3.36 L£¬ŹĒ³É±ČĄżŌö¼ÓµÄ£¬ĖµĆ÷NaOHČÜŅŗ“ĖŹ±ŹĒ×ćĮæµÄ£®ĖłŅŌæÉĶʶĻ³öµ±ŃłĘ·µÄÖŹĮæĪŖ3.7 gŹ±£¬ĖłŹĶ·Å³öµÄĘųĢåĢå»żÓ¦ĪŖ£ŗ1.68 L”Ā2£½0.84 L£® ””””(3)Éč¢ń×é»ģŗĻĪļÖŠ(NH4)2SO4µÄĪļÖŹµÄĮæĪŖx£¬NH4HSO4µÄĪļÖŹµÄĮæĪŖy£¬ŅĄ¾Ż±ķÖŠµÄŹż¾ŻÓŠ£ŗµ±»ģŗĻĪļÖŹĮæĪŖ14.8 gŹ±£¬²śÉśNH3µÄĪļÖŹµÄĮæĪŖ£ŗ
|



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
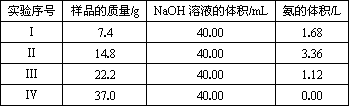
ĪŖ²ā¶ØÉĻŹö£ØNH4£©2SO4ŗĶNH4HSO4¹ĢĢå»ģŗĻĪļµÄ×é³É£¬ĻÖ³ĘČ”øĆѳʷ4·Ż£¬·Ö±š¼ÓČėĻąĶ¬ÅØ¶ČµÄNaOHČÜŅŗø÷40.00 mL£¬¼ÓČČÖĮ120 ”ę×óÓŅ£¬Ź¹°±ĘųČ«²æŅŻ³ö”²£ØNH4£©2SO4ŗĶNH4HSO4¹ĢĢå·Ö½āµÄĪĀ¶Č¾łøßÓŚ200 ”ę”³£¬²āµĆÓŠ¹ŲŹµŃ鏿¾ŻČēĻĀ£ŗ£Ø±ź×¼×“æöĻĀ£©
ŹµŃéŠņŗÅ | ѳʷµÄÖŹĮæ/g | NaOHČÜŅŗµÄĢå»ż/mL | °±ĘųµÄĢå»ż/L |
¢ń | 7.4 | 40.00 | 1.68 |
¢ņ | 14.8 | 40.00 | 3.36 |
¢ó | 22.2 | 40.00 | 1.12 |
¢ō | 37.0 | 40.00 | 0.00 |
£Ø1£©ŹµŃé¹ż³ĢÖŠÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_________________________________________”£
£Ø2£©ÓÉ¢ń×鏿¾ŻÖ±½ÓĶĘ²ā£ŗ±ź×¼×“æöĻĀ3.7 g ѳʷ½ųŠŠĶ¬ŃłŹµŃ鏱£¬Éś³É°±ĘųµÄĢå»żĪŖ__________L”£
£Ø3£©ŹŌ¼ĘĖćøĆ»ģŗĻĪļÖŠ£ØNH4£©2SO4ŗĶNH4HSO4µÄĪļÖŹµÄĮæÖ®±ČĪŖ__________________”£
£Ø4£©Óū¼ĘĖćøĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČÓ¦øĆŃ”Ōń__________×鏿¾Ż£¬ÓÉ“ĖĒóµĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖĮĖŌ¤·Ą»·¾³ĪŪČ¾²¢¶ŌĪ²Ęų½ųŠŠ×ŪŗĻĄūÓĆ£¬Ä³ĮņĖį³§ÓĆ°±Ė®ĪüŹÕĪ²ĘųÖŠµÄSO2”£ŌŁĻņĪüŹÕŅŗÖŠ¼ÓČėÅØĮņĖį£¬ŅŌÖĘČ”øßÅØ¶ČµÄSO2¼°£ØNH4£©2SO4ŗĶNH4HSO4¹ĢĢ唣
ĪŖ²ā¶ØÉĻŹö£ØNH4£©2SO4ŗĶNH4HSO4¹ĢĢå»ģŗĻĪļµÄ×é³É£¬ĻÖ³ĘČ”øĆѳʷ4·Ż£¬·Ö±š¼ÓČėĻąĶ¬ÅØ¶ČµÄNaOHČÜŅŗø÷40.00 mL£¬¼ÓČČÖĮ120 ”ę×óÓŅ£¬Ź¹°±ĘųČ«²æŅŻ³ö”²£ØNH4£©2SO4ŗĶNH4HSO4¹ĢĢå·Ö½āµÄĪĀ¶Č¾łøßÓŚ200 ”ę”³£¬²āµĆÓŠ¹ŲŹµŃ鏿¾ŻČēĻĀ£ŗ£Ø±ź×¼×“æöĻĀ£©
| ŹµŃéŠņŗÅ | ѳʷµÄÖŹĮæ/g | NaOHČÜŅŗµÄĢå»ż/mL | °±ĘųµÄĢå»ż/L |
| ¢ń | 7.4 | 40.00 | 1.68 |
| ¢ņ | 14.8 | 40.00 | 3.36 |
| ¢ó | 22.2 | 40.00 | 1.12 |
| ¢ō | 37.0 | 40.00 | 0.00 |
£Ø1£©ŹµŃé¹ż³ĢÖŠÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_________________________________________”£
£Ø2£©ÓÉ¢ń×鏿¾ŻÖ±½ÓĶĘ²ā£ŗ±ź×¼×“æöĻĀ3.7 g ѳʷ½ųŠŠĶ¬ŃłŹµŃ鏱£¬Éś³É°±ĘųµÄĢå»żĪŖ__________L”£
£Ø3£©ŹŌ¼ĘĖćøĆ»ģŗĻĪļÖŠ£ØNH4£©2SO4ŗĶNH4HSO4µÄĪļÖŹµÄĮæÖ®±ČĪŖ__________________”£
£Ø4£©Óū¼ĘĖćøĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČÓ¦øĆŃ”Ōń__________×鏿¾Ż£¬ÓÉ“ĖĒóµĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ____________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com