
 �������ͼ����
�������ͼ����| 162-32 |
| 13 |
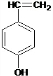 ��BΪ
��BΪ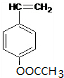 ���ݴ˽��
���ݴ˽��| 162-32 |
| 13 |
 ��BΪ
��BΪ ��
��| �� |
| �� |
 ����һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ��
����һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ��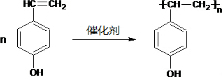 ��
�� ��
��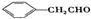 ��
�� ��
��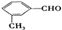 ��
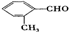
�� ��4�֣����в�������ͬ���칹��Ľṹ��ʽΪ
��4�֣����в�������ͬ���칹��Ľṹ��ʽΪ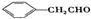 ���ʴ�Ϊ��4��
���ʴ�Ϊ��4��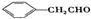 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1.8g ˮ�����еĵ�����ĿΪNA |
| B��2g ��������ԭ����ΪNA |
| C�����³�ѹ��11.2L��������������ĿΪ0.5NA |
| D��200 mL0.5mol?L-1Na2SO4��Һ����Na+��Ŀ0.1NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ʵ���� | �Թܢ����Լ� | �Թܢ����л���ĺ��/cm |
| A | 2mL�Ҵ���1mL���ᡢ1mL 18mol?L-1Ũ���� | 3.0 |
| B | 2mL�Ҵ���1mL���� | 0.1 |
| C | 2mL�Ҵ���1mL���� 6mL 3mol?L-1 H2SO4 | 0.6 |
| D | 2mL�Ҵ���1mL���ᡢ���� | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
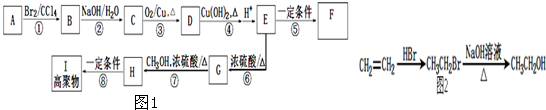
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH4��C2H4 |
| B��C2H2��C3H8 |
| C��C2H4��C3H4 |
| D��C2H2��C3H6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NaOH��Һ����ε���Fe2��SO4��3��Һֱ������ |
| B����NaOH��Һ��ε���AlCl3��Һ�У�ֱ������ |
| C����AlCl3��Һ����ε���ϡ���� |
| D������ˮ��ε�����������Һ�У�ֱ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ͬ��ͬѹ�£����ʵ����ʵ�����ȣ������һ����ͬ |
| B��������Ķ�����̼��һ����̼�����ķ�����һ����� |
| C��1L����һ����1L����������С |
| D����ͬ�����µ�һ����̼����͵������������ȣ�������һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
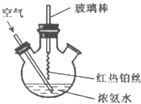
| A����Ӧ����Һ�к���NO3- |
| B����Ӧ����Һ��c��H+������ |
| C��ʵ��������л��Ϸ�Ӧ���� |
| D��ʵ�������NH3?H2O�ĵ��볣�������ܷ����仯 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com