
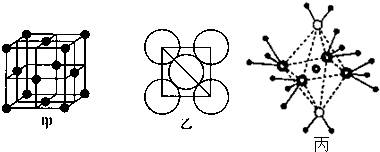
���� ��1������ͭ��Ԫ�����ڱ��е�λ������д��̬ԭ�ӵ����Ų�ʽ��ȷ��������
��2��Cu2O��Cu2Sͬ�������Ӿ��壬���Ӿ����۵�ĸߵ�Ҫͨ�������ܵĴ�С���жϣ�
��3��������λ���������ΪCO������ԭ�ӽ�ϳɽ������壻
��4������ԭ���ṩ�չ����ͨ������Al�۲���Ӷ������ӻ����ͣ�
��5������ͼ�ҿ�����ѻ���ʽΪABCABC������ͨ����ÿ����������������������ܶȣ�
��6����5��Ԫ���γɵ�һ��1��1�����ӻ������У������ӳ�������ṹ��˵���������Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ������ӳ����������İ�����ṹ������ͼ֪������������ͭ������λ����8���ڰ��������¶����Ϸ��Ӻ����������ۼ��Һ��������µ��Ӷԣ�Ϊˮ���ӣ���������������ƽ�����ĸ������Ϸ��Ӻ���3�����ۼ��Һ���һ���µ��Ӷԣ�����Ϊ�������ӣ���4�����������д��ڹ��ۼ�����λ����H2O��Cu2+����λ����NH3��Cu2+���������Ըû��������ʱ����ʧȥ�������H2O��
��� �⣺��1��ͭ��29��Ԫ�أ�λ�ڵ������ڢ�B�壬��Χ�����Ų�Ҫ��ѭ���ع�������Ϊ3d104s1���ʴ���ds���������Ų�Ϊ1s22s22p63s23 p63d104s1��[Ar]3d104s1��
�ʴ�Ϊ��ds��1s22 s22p63 s23 p63d104s1��[Ar]3d104s1��
��2��Cu2O��Cu2Sͬ�������Ӿ��壬����������ͬ�������������ĵ��Ҳ��ͬ���������ӵİ뾶С�������ӵ����Ӱ뾶������Cu2O�ľ����ܴ��۵�ߣ�
�ʴ�Ϊ��Cu2O��Cu2S��Ƚϣ�����������ͬ�������������ĵ��Ҳ��ͬ�������������ӵİ뾶С�������ӵ����Ӱ뾶��Cu2O�ľ����ܸ�����ͭ�������������γɵ����Ӽ�ǿ����ͭ�������������γɵ����Ӽ���������Cu2O���۵��Cu2S�ĸߣ�
��3�������������ԭ��Ϊ����ԭ�ӣ����ڶ��ѵ�������ԭ�Ӻ�����֮�����λ�������Զ��Ѻ������γ�CO������ԭ�Ӽ��γɽ�������Ϊ�������壬
�ʴ�Ϊ����������
��4��AlCl3•NH3��AlΪ����ԭ�ӣ�NH3��Cl-Ϊ���壬����ԭ���ṩ�չ����AlCl4-�ļ۲���Ӷ�=4+$\frac{1}{2}$��3+1-4��1��=4����Al����sp3�ӻ���
�ʴ�Ϊ��Al��sp3��
��5�����ͼ�Һ;����ṹ��֪����������Al�Ķѻ���ʽΪABCABCABC�������������������ܶѻ���
��ԭ�Ӱ뾶Ϊd cm�����ı���Ϊ2$\sqrt{2}$dcm�����������V=��2$\sqrt{2}$dcm��3=16$\sqrt{2}$d3cm3��һ����������Alԭ�Ӹ���Ϊ��8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��һ������������m=$\frac{4}{{N}_{A}}$��Mg�����ܶ�$��=\frac{m}{V}$=$\frac{\frac{4M}{{N}_{A}}}{16\sqrt{2}{d}^{3}}$g/cm3=$\frac{M}{4\sqrt{2}{N}_{A}{d}^{3}}$g/cm3��
�ʴ�Ϊ�������������ܶѻ���$\frac{M}{4\sqrt{2}{N}_{A}{d}^{3}}$g/cm3��
��6����5��Ԫ���γɵ�һ��1��1�����ӻ������У������ӳ�������ṹ��˵���������Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�ӦΪSO42-��
�����ӳ����������İ�����ṹ����ͼ2��ʾ����������ΪCu2+����λ����6���ڰ��������¶����Ϸ��Ӻ����������ۼ��Һ��������µ��Ӷԣ�ΪH2O���ӣ���2����������ƽ�����ĸ������Ϸ��Ӻ���3�����ۼ��Һ���һ���µ��Ӷԣ�����ΪNH3���ӣ���4�����仯ѧʽΪ[Cu��NH3��4��H2O��2]SO4��H2O��Cu2+����λ����NH3��Cu2+���������Ըû��������ʱ����ʧȥ�������H2O��
�ʴ�Ϊ��SO42-��H2O��H2O��Cu2+����λ����NH3��Cu2+������
���� ���⿼�����ʽṹ�����ʣ����ؿ���ѧ���ռ�����������֪ʶ�����������漰��̬�����Ų�ʽ���۵�Ƚϡ��������㡢����ԭ�ӽṹ��֪ʶ�㣬�ۺ��Խ�ǿ�����þ�̯�����۲���ӶԻ������۵����۷��������Ŀ�Ѷ��еȣ�ע�⣺��λ��Ҳ���ڹ��ۼ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������ȣ�Li-SOCl2����ؾ��������ܶȸߡ�������ѹ�ͷŵ��ѹƽ�ȡ������¶ȷ�Χ�����������������ŵ㣬�ں�����ҽ�Ƽ����������豸�ȷ����Ӧ�ù㷺��
��������ȣ�Li-SOCl2����ؾ��������ܶȸߡ�������ѹ�ͷŵ��ѹƽ�ȡ������¶ȷ�Χ�����������������ŵ㣬�ں�����ҽ�Ƽ����������豸�ȷ����Ӧ�ù㷺���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
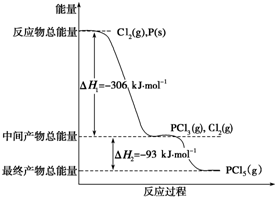 ��֪����P��s����Cl2��g��������Ӧ����PCl3��g����PCl5��g������Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���
��֪����P��s����Cl2��g��������Ӧ����PCl3��g����PCl5��g������Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ�Ⱦ�Ϊ0.1mol/L��HCN��Һ��NaCN��Һ�������Ϻ���Һ�ʼ���c��CN-����c��Na+����c��HCN����c��OH-����c��H+�� | |
| B�� | pH=a�Ĵ�����Һ��ϡ��100������pH=b����a+2��b | |
| C�� | c��NH4+����ͬ�Ģ��Ȼ�梨�������梨۴�����梨�̼���������Һ��pH���ܣ��ڣ��٣��� | |
| D�� | pH=5��H2S��Һ�У�c��HS-����c��H+��=1��10-5mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
�� ��
�� ������һ�֣�����д�����е�һ�֣�
������һ�֣�����д�����е�һ�֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ��Ӧ���������µ������⣬�������������ı仯 | |
| B�� | ���ʵ�ȼ��һ���Ƿ��ȷ�Ӧ | |
| C�� | ���ȵĻ�ѧ��Ӧ����Ҫ���Ⱦ��ܷ��� | |
| D�� | ��ѧ�������֮������ת�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com