


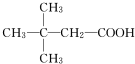
 £®£ØÓĆ½į¹¹¼ņŹ½±ķŹ¾£©
£®£ØÓĆ½į¹¹¼ņŹ½±ķŹ¾£© ·ÖĪö ÓÉ×Ŗ»Æ¹ŲĻµæÉÖŖ£¬Eŗ¬ÓŠōČ»ł”¢Gŗ¬ÓŠ-OH£¬ĒŅE”¢Gŗ¬ÓŠĻąĶ¬µÄĢ¼Ō×ÓŹżÄ棬¹ŹEÓėG·¢Éśõ„»Æ·“Ӧɜ³ÉH£¬ÓÉHµÄ·Ö×ÓŹ½æÉÖŖ£¬EĪŖCH3CH2COOH£¬FÖŠŗ¬ÓŠ2øö¼×»ł£¬ŌņGĪŖCH3CH£ØOH£©CH3£¬ÄęĶĘæÉµĆ£¬FĪŖCH3CHClCH3£¬DĪŖCH3CH2CHO£¬CĪŖCH3CH2CH2OH£¬BĪŖCH3CH2CH2Cl£¬AĪŖCH3CH=CH2£¬ŅŌ“Ė½ā“šøĆĢā£®
½ā“š ½ā£ŗ£Ø1£©ÓÉÉĻŹö·ÖĪöæÉÖŖ£¬DµÄ½į¹¹¼ņŹ½ĪŖCH3CH2CHO£¬
¹Ź“š°øĪŖ£ŗCH3CH2CHO£»
£Ø2£©ĢžA”śBµÄ»Æѧ·“Ó¦·½³ĢŹ½ŹĒ£ŗCH3CH=CH2+HCl$\stackrel{Ņ»¶ØĢõ¼ž}{”ś}$CH3CH2CH2Cl£¬
¹Ź“š°øĪŖ£ŗCH3CH=CH2+HCl$\stackrel{Ņ»¶ØĢõ¼ž}{”ś}$CH3CH2CH2Cl£»
£Ø3£©F”śGŹĒCH3CHClCH3·¢ÉśČ”“ś·“Ӧɜ³ÉCH3CH£ØOH£©CH3£¬Éś³ÉĪļĪŖ2-±ū“¼£¬
¹Ź“š°øĪŖ£ŗČ”“ś·“Ó¦£»2-±ū“¼£»
£Ø4£©E+G”śHµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ£ŗCH3CH2COOH+CH3CH£ØOH£©CH3$”ś_{”÷}^{ÅØĮņĖį}$CH3CH2COOCH£ØCH3£©2+H2O£¬
¹Ź“š°øĪŖ£ŗCH3CH2COOH+CH3CH£ØOH£©CH3$”ś_{”÷}^{ÅØĮņĖį}$CH3CH2COOCH£ØCH3£©2+H2O£»
£Ø5£©HÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬ĘäÖŠŗ¬ÓŠŅ»øöōČ»ł£¬ĒŅĘäŅ»ĀČ“śĪļÓŠĮ½ÖֵďĒ £¬
£¬
¹Ź“š°øĪŖ£ŗ £®
£®
µćĘĄ ±¾Ģā×ŪŗĻæ¼²éÓŠ»śĪļµÄĶʶĻ£¬ĪŖøßæ¼³£¼ūĢāŠĶ£¬²ąÖŲæ¼²éÓŠ»śĪļµÄŠŌÖŹ”¢Ķ¬·ÖŅģ¹¹Ģ唢ӊ»ś·“Ó¦ĄąŠĶµČ£¬ÄŃ¶Č²»“ó£¬ÕĘĪÕ¹ŁÄÜĶŵĊŌÖŹŌ½×Ŗ»ÆŹĒ¹Ų¼ü£¬×¢Ņāøł¾ŻHµÄ·Ö×ÓŹ½ĄūÓĆÄęĶĘ·Ø½ųŠŠĶʶĻ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ņ»¶Øŗ¬Ag+ | B£® | Ņ»¶Øŗ¬SO42- | C£® | ŗ¬ÓŠAg+»ņSO42- | D£® | ŗ¬ÓŠAg+ŗĶSO42- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | µē½āĮņĖį»ņĖįŹ½ĮņĖįŃĪČÜŅŗ£¬Ńō¼«·“Ó¦ĪŖ2SO42--2e-ØTS2O82- | |
| B£® | S2O82-¾ßÓŠĒæŃõ»ÆŠŌ£¬H2S2O8ŹĒ¶žŌŖĒæĖį | |
| C£® | ¼õŃ¹ÕōĮóµÄÄæµÄŹĒĪŖĮĖ¼õÉŁH2O2·Ö½ā£¬ÕōĮóµĆµ½µÄĮķŅ»×é·ÖæÉŃ»·ĄūÓĆ | |
| D£® | ÓÉČÜŅŗA×Ŗ»ÆĪŖČÜŅŗB·¢ÉśĮĖŃõ»Æ»¹Ō·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CuSO4 | B£® | H2SO4 | C£® | H2O | D£® | SO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2SO4 | B£® | CH3COOH | C£® | £ØNH4£©2 SO4 | D£® | NaOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na+ | B£® | Al3+ | C£® | Fe2+ | D£® | Fe3+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
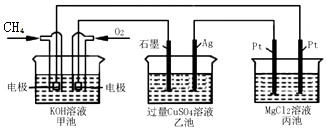
| A£® | ¼×³ŲŹĒµēÄÜ×Ŗ»ÆĪŖ»ÆѧÄܵÄ×°ÖĆ£¬ŅŅ”¢±ū³ŲŹĒ»ÆѧÄÜ×Ŗ»ÆµēÄܵÄ×°ÖĆ | |
| B£® | ¼×³ŲÖŠÕż¼«µÄµē¼«·“Ó¦Ź½ŹĒO2+4e-+4H+=2H2O | |
| C£® | ·“Ó¦¹ż³ĢÖŠ£¬ŅŅ³ŲµÄpHÖš½„¼õŠ” | |
| D£® | ¼×³ŲÖŠĻūŗÄO2µÄĢå»żÓė±ū³ŲÉś³ÉĘųĢåµÄ×ÜĢå»żŌŚĻąĶ¬Ģõ¼žĻĀµÄ±ČÖµĪŖ1£ŗ2 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com