
ijͬѧ��������װ��ʵ��ͭ��Ũ���ᡢϡ���ᷴӦ���������£�
I�� ȡһ��ͭ˿����ϡ�����ȥͭ��[��Ҫ�ɷ���Cu2(OH)2CO3]��
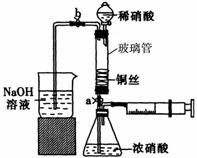 ��ϴ�Ӻ��ͭ˿���������϶������״��
��ϴ�Ӻ��ͭ˿���������϶������״��
III������ͼ��ʾװ��������������������ԡ�װ�뻯ѧ�Լ���
(1)����I������Ӧ�����ӷ���ʽ�� ��
(2)д������III�м�������Եķ��� ��
�ǹ���III�ĺ����������£�
�ٴ�ֹˮ��a��b������ע������ʹŨ������ͭ˿�Ӵ����۲쵽�������� ��һ��ʱ���ʹ��Ӧֹͣ�IJ����� ���ر�a��ȡ��ע������
�ڴ�b�ͷ�Һ©���������������ܳ���ϡ����ر�b�ͷ�Һ©����������a���۲쵽�����ݲ�����ϡ������������ܵ�ʵ��Ŀ�� ���÷�Ӧ�����ӷ���ʽ�� ��
(4)��ȡ3֧ʢ��NO2�����С�Թֱܷ�����ʢ�г���ˮ����ˮ�ͱ�ˮ��3ֻ�ձ��У�����Һ�������ĸ߶����Բ�һ�¡�������±���ʾ(�����¶ȶ����������Ӱ��)��ks5u

�ٸ����ϱ��ó��Ľ������¶�Խ (��ߡ��͡�)�������Թ��е���ҺԽ�ࡣ
�ڲ������ϣ�a��NO2��ˮ��Ӧ��ʵ�ʹ���Ϊ��2NO2+H2O=HNO3+HNO2
3HNO2 =HNO3+2NO+H2O�� b��HNO2���ȶ���
��������������ԭ���� ��
��1��Cu2(OH)2CO3+4H+==2Cu2++CO2��+3H2O
��2����b��a���رշ�Һ©���Ļ���������ߵ��ܲ���ʢˮ���ձ��У���������ע������������������Һ��������˵�������Ժá������Ʒ�������ȷ��
��3���ٲ�������ɫ���� ���Ὣע��������������ʹͭ˿����Һ�ֿ�
�ڽ��������е�NO2�Ϳ����ų� 3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
��4���ٵͣ�
���¶ȵͣ�HNO2�ֽ������٣��ֽ������NO���������٣����Խ����Թܵ���Һ��


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ����ʮ�и���5���¿������ۣ���ѧ���� ���ͣ�ʵ����
��15�֣���������ͭ��ͭ����ɵĻ���ijͬѧ������ͼ��ʾװ�ã�ͨ���ⶨ �����������ʵ��ǰ��U�������仯��ȷ�������������ͭ������������
�����������ʵ��ǰ��U�������仯��ȷ�������������ͭ������������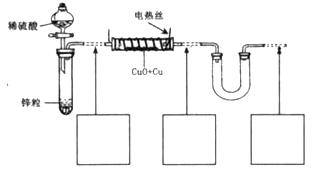
�ش��������⣺
��1��U�ι��п��Լ����������_________������ţ���
A��Ũ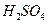 B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
��2�����в��谴ʵ�����˳��ӦΪ_________������ĸ����
A��ֹͣͨ������ B������˿ͨ�磻 C��ͨ��������
D��װ�������Լ�飻 E������˿ֹͣͨ�硣
��3��Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�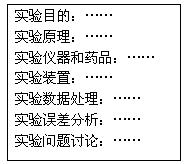
��4��ʵ�������ͬѧ������ʦ��ʵ�鱨����Ҫ��Ŀ��ͼ�������������ԣ����������ʵ�鱨���дҪ�Դ˷ݱ����������ۣ�������������������д��������������������Ŀո���д����ȱ��Ŀ______ _
��5����ʦ����ʵ�鱨���ָ�����ı�ʵ��ԭ��������Ƴ����Ӽ���ʵ�鷽�������û�ѧ����ʽ��ʾ����Ƶ��·����ķ�Ӧԭ��____ ______���÷�����ⶨ������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ������ѧ�ٷ�һ������һ�г��ζ��и�����������У���������ۣ���ѧ�� ���ͣ������
��������ͭ��ͭ����ɵĻ���ijͬѧ������ͼ��ʾװ�ã�ͨ���ⶨ�����������ʵ��ǰ��U�������仯��ȷ�������������ͭ������������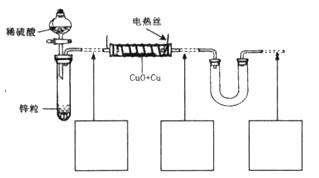
�ش��������⣺
��1��U�ι��п��Լ����������_________������ţ���
A��Ũ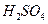 B����ˮ����ͭ�� C����ˮ�Ȼ��ƿ���
B����ˮ����ͭ�� C����ˮ�Ȼ��ƿ���
��2�����в��谴ʵ�����˳��ӦΪ_________������ĸ����
A��ֹͣͨ������ B������˿ͨ�磻 C��ͨ��������
D��װ�������Լ�飻 E������˿ֹͣͨ�硣
��3��Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�
��4��ʵ�������ͬѧ������ʦ��ʵ�鱨����Ҫ��Ŀ��ͼ�������������ԣ����������ʵ�鱨���дҪ�Դ˷ݱ����������ۣ�������������������д��������������������Ŀո���д����ȱ��Ŀ______ _______ __��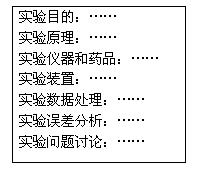
��5����ʦ����ʵ�鱨���ָ�����ı�ʵ��ԭ��������Ƴ����Ӽ���ʵ�鷽�������û�ѧ����ʽ��ʾ����Ƶ��·����ķ�Ӧԭ��____ ________��
�÷�����ⶨ������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и���4�¹�����ѵ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
����(Na2CO3)�����������о��й㷺����;��������ʵ����ģ���Ƽ�ԭ����ȡNa2CO3������ͼ

��֪:��ʳ��ˮ��ͨ��NH3��CO2�����ͷ�ӦΪNaCl��NH3��CO2��H2O NaHCO3����NH4Cl,��ش��������⣺
NaHCO3����NH4Cl,��ش��������⣺
��1�������к��е�����������Ca2+��Mg2+��SO42-�ȡ�
���Ƴ��ӵIJ���˳��a��_______��________��________��b(����ĸ��ţ���
a�������ܽ⣬��ȥ������b�����������pH��c������Ba(OH)2��Һ��d������Na2CO3��Һ��e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2��������_____________________��
��2�����չ���A��Na2CO3��_____����ĸ��ţ��н��С�
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4+�ķ�����__________________________________________________________��
����ҺA�����ؽᾧ�ܹ����NH4HCO3����pH=13��Na+��K+����Һ�м�������NH4HCO3��ʹpH���ͣ���Ӧ�����ӷ���ʽ____________________________________��
��3����ͼװ���г�����ʵ�����Ʊ�CO2����_____(����ĸ��ţ�����bʾ���װ���Ʊ�NH3����Һ©����ʢ�ŵ��Լ�______(���Լ����ƣ�����ƿ�ڿɼ���Ĺ����Լ�__________�����Լ����ƣ���

��4��һ����Ȼ���ɷ���aNa2CO3��bNa2CO3��cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ����������������ѡ�����ʵ�鷽����ȫ����ѡ����Լ���1.0mol/LH2SO4��Һ��1.0mol/L BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca(OH)2��Һ������ˮ
�ٳ�ȡm1gһ������Ȼ�����Ʒ��������������ˮ�С�
��_________________________________________________________________��
��_________________________________________________________________��
�ܼ�����Ȼ����к�Na2CO3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ����5���¿������ۣ���ѧ���� ���ͣ�ʵ����
��15�֣���������ͭ��ͭ����ɵĻ���ijͬѧ������ͼ��ʾװ�ã�ͨ���ⶨ�����������ʵ��ǰ��U�������仯��ȷ�������������ͭ������������
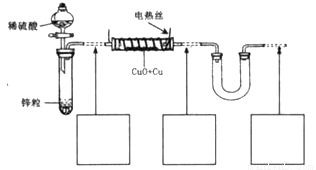
�ش��������⣺
��1��U�ι��п��Լ����������_________������ţ���
A��Ũ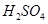 B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
��2�����в��谴ʵ�����˳��ӦΪ_________������ĸ����
A��ֹͣͨ������ B������˿ͨ�磻 C��ͨ��������
D��װ�������Լ�飻 E������˿ֹͣͨ�硣
��3��Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�

��4��ʵ�������ͬѧ������ʦ��ʵ�鱨����Ҫ��Ŀ��ͼ�������������ԣ����������ʵ�鱨���дҪ�Դ˷ݱ����������ۣ�������������������д��������������������Ŀո���д����ȱ��Ŀ______ _
��5����ʦ����ʵ�鱨���ָ�����ı�ʵ��ԭ��������Ƴ����Ӽ���ʵ�鷽�������û�ѧ����ʽ��ʾ����Ƶ��·����ķ�Ӧԭ��____ ______���÷�����ⶨ������______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com