
£Ø1£©Ę½ŗāŹ±N2O5£Øg£©ŗĶN2O£Øg£©µÄÅØ¶Č”£
£Ø2£©“Ó·“Ó¦æŖŹ¼µ½“ļµ½Ę½ŗāŹ±N2O5£Øg£©µÄĘ½¾ł·“Ó¦ĖŁĀŹ”£
£Ø1£©c(N2O5)=0.009 4 mol”¤L-1£¬c(N2O)=0.014 4 mol”¤L-1
£Ø2£©v(N2O5)=0.006 12 mol”¤L-1”¤min-1
½āĪö£ŗÉč“ļµ½Ę½ŗāŹ±ĻūŗĵÄN2O5£Øg£©ĪļÖŹµÄĮæÅضČĪŖx£¬Éś³ÉµÄN2O£Øg£©ĪļÖŹµÄĮæÅضČĪŖy£¬Ōņ
N2O5£Øg£© ![]() N2O3(g) + O2(g)
N2O3(g) + O2(g)
æŖŹ¼£ŗ0.040 0 mol”¤L-1 0 0
Ę½ŗā£ŗ0.040 0 mol”¤L-1-x x x
N2O3(g)![]() N2O(g)+O2(g)
N2O(g)+O2(g)
Ę½ŗā£ŗx-y y y
ŅĄĢāŅāµĆ£ŗ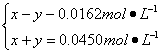
½āµĆ
ĖłŅŌ£¬£Ø1£©Ę½ŗāŹ±c(N2O5)=0.040 0 mol”¤L-1-0.030 6 mol”¤L-1=0.009 4 mol”¤L-1,c(N2O)=0.014 4 mol”¤L-1”£
![]()


 ČŹ°®Ó¢ÓļĶ¬²½Į·Ļ°²įĻµĮŠ“š°ø
ČŹ°®Ó¢ÓļĶ¬²½Į·Ļ°²įĻµĮŠ“š°ø ѧĻ°Źµ¼łŌ°µŲĻµĮŠ“š°ø
ѧĻ°Źµ¼łŌ°µŲĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com