
ij����С��������ʵ�飺��һ�����������ڳ���CO2��Ȼ��������ע��������NaOH��Һ�������ý����Ϸ�ڣ���һ��ʱ������Ƿ��ֹޱ��ڰ������ٹ�һ��ʱ����˵Ĺޱ����¹��𣬽�������ʵ������ı仯��
(1)�ޱ��ڰ������ԭ����________����Ӧ�Ļ�ѧ����ʽ________��
(2)�ޱ��ٹ����ԭ����________���й����ӷ���ʽ________��
(3)����ý�������Ƥ������������ʵ����ֵĽ����________��
 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ij����С���������¹��ڼص�̽��ʵ�飮
ij����С���������¹��ڼص�̽��ʵ�飮| ������� | �����Լ� | ������ | ����� |
| A | MnO2��ϡ���� | ����ʳ��ˮ | Ũ���� |
| B | Ca��ClO��2��Ũ���� | ����ʳ��ˮ | ��ˮ����ͭ |
| C | ����ء�Ũ���� | ����̼������Һ | Ũ���� |
| D | KMnO4��Ũ���� | ˮ | ��ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���¿αꡡ�̲�ȫ�⡡���л�ѧ�����˽̰�(����1) �˽̰� ���ͣ�022
ij����С��������ʵ�飺��һ�����������ڳ���CO2��Ȼ��������ע��������NaOH��Һ�������ý����Ϸ�ڣ���һ��ʱ������Ƿ��ֹޱ��ڰ������ٹ�һ��ʱ����˵Ĺޱ����¹��𣬽��������仯��ʵ������
(1)�ޱ��ڰ������ԭ����________����Ӧ�Ļ�ѧ����ʽ��________��
(2)�ޱ��ٹ����ԭ����________���йصĻ�ѧ����ʽ��________��
(3)����ý�������������������ʵ����ֵĽ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
ij����С��������ʵ�飺��һ�����������ڳ���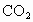 ��Ȼ��������ע��������NaOH��Һ�������ý����Ϸ�ڣ���һ��ʱ������Ƿ��ֹޱ��ڰ������ٹ�һ��ʱ����˵Ĺޱ����¹��𣬽�������ʵ������ı仯��
��Ȼ��������ע��������NaOH��Һ�������ý����Ϸ�ڣ���һ��ʱ������Ƿ��ֹޱ��ڰ������ٹ�һ��ʱ����˵Ĺޱ����¹��𣬽�������ʵ������ı仯��
(1)�ޱ��ڰ������ԭ����__________����Ӧ�Ļ�ѧ����ʽ__________��
(2)�ޱ��ٹ����ԭ����__________���й����ӷ���ʽ__________��
(3)����ý�������Ƥ������������ʵ����ֵĽ����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���ޱ��ڰ������ԭ����_______����Ӧ�Ļ�ѧ����ʽΪ_______________________��
��2���ޱ��ٹ����ԭ����_______���йػ�ѧ����ʽΪ____________________________��
��3������ý���������������������ʵ�飬���ֵĽ����_________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com