
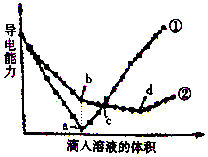
 ��������ͬ��Ba��OH��2��Һ�У��ֱ�������ʵ���Ũ����ȵ�H2SO4��NaHSO4��Һ���䵼�������������Һ����仯��������ͼ��ʾ��
��������ͬ��Ba��OH��2��Һ�У��ֱ�������ʵ���Ũ����ȵ�H2SO4��NaHSO4��Һ���䵼�������������Һ����仯��������ͼ��ʾ������ Ba��OH��2��Һ��H2SO4��NaHSO4��Һ��Ӧ����ʽ�ֱ�ΪH2SO4+Ba��OH��2=BaSO4��+2H2O��NaHSO4+Ba��OH��2=BaSO4��+NaOH+H2O��2NaHSO4+Ba��OH��2=BaSO4��+Na2SO4+2H2O����Һ��������������Ũ�ȳ����ȣ�����ͼ֪�����ߢ���a����Һ���������ӽ�0��˵���õ���Һ����Ũ����С��Ӧ��ΪBa��OH��2��Һ��H2SO4�ķ�Ӧ�������ߢ�ΪBa��OH��2��Һ��NaHSO4��Һ�ķ�Ӧ������ͼ֪��a��ΪBa��OH��2��Һ��H2SO4ǡ�÷�Ӧ��H2SO4��NaHSO4��Һ�����ʵ���Ũ����ȣ���b����Һ����ΪNaOH��c�㣬����ϡ�������������Ϊ���ᣬ���з�Ӧ������ΪNaOH��Na2SO4��a������������������ǡ����ȫ��Ӧ����Һ��ֻ��ˮ��d���������ΪNa2SO4��
��� �⣺Ba��OH��2��Һ��H2SO4��NaHSO4��Һ��Ӧ����ʽ�ֱ�ΪH2SO4+Ba��OH��2=BaSO4��+2H2O��NaHSO4+Ba��OH��2=BaSO4��+NaOH+H2O��2NaHSO4+Ba��OH��2=BaSO4��+Na2SO4+2H2O����Һ��������������Ũ�ȳ����ȣ�����ͼ֪�����ߢ���a����Һ���������ӽ�0��˵���õ���Һ����Ũ����С��Ӧ��ΪBa��OH��2��Һ��H2SO4�ķ�Ӧ�������ߢ�ΪBa��OH��2��Һ��NaHSO4��Һ�ķ�Ӧ�����ٴ����μ�H2SO4��Һ�ı仯���ߣ�
��1��NaHSO4��ǿ����ʣ�����Һ�У�NaHSO4�ĵ��뷽��ʽΪNaHSO4=Na++H++SO42-��
�ʴ�Ϊ��NaHSO4=Na++H++SO42-��
��2�����ߢ�ΪBa��OH��2��Һ��NaHSO4��Һ�ķ�Ӧ���ٴ����μ�H2SO4��Һ�ı仯���ߣ��ʴ�Ϊ��H2SO4��NaHSO4��
��3��a��ΪBa��OH��2��Һ��H2SO4ǡ�÷�Ӧ��H2SO4��NaHSO4��Һ�����ʵ���Ũ����ȣ���b����Һ����ΪNaOH���ʴ�Ϊ��Na+��OH-��
��4��a��ΪBa��OH��2��Һ��H2SO4ǡ�÷�Ӧ�������ᱵ��ˮ����ʾ���ԣ�H2SO4��NaHSO4��Һ�����ʵ���Ũ����ȣ���b����Һ����ΪNaOH����ʾ���ԣ�
�ʴ�Ϊ�����ԣ����ԣ�
��5��d���������ΪNa2SO4����ʱ������Ӧ��Ba��OH��2+2NaHSO4=BaSO4��+Na2SO4+2H2O�������ƶ�����Ũ����С�����������������ʴ�Ϊ��Ba��OH��2+2NaHSO4=BaSO4��+Na2SO4+2H2O��
��6��c�㣬����ϡ�������������Ϊ���ᣬ���з�Ӧ������ΪNaOH��Na2SO4������Һ�к�����ͬ����SO42-���ʴ�Ϊ��SO42-��
���� ���⿼���������Һ�����жϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������жϼ�ʶͼ��������ȷ�����ķ�Ӧ��������Һ�����ʳɷ��ǽⱾ��ؼ���ע�⣺��Һ��������������Ũ�ȳ����ȣ���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȡˮ��Ͳ�����ԭ���о��д��� | |
| B�� | ��ͨ�����ǵ�ľ�Ե�壬�������Ĺ��������ǻ����̶����۵� | |
| C�� | ��ͨ�����׳�ˮ���� | |
| D�� | �����ι�ҵʹ�õ�ÿһ��ԭ�϶����й� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ȼ�ϵ�������Խ����е�������Ӧʽ��O2+4H++4e-�T2H2O | |
| B�� | ��������������̼����������ⱥ���Ȼ�����Һ�����ӷ���ʽΪ��2C1-+2H2O$\frac{\underline{\;���\;}}{\;}$H2��+Cl2��+2OH- | |
| C�� | ��ͭ����ʱ�����Դ�����������Ǵ�ͭ���缫��ӦʽΪ��Cuһ2e-=Cu2+ | |
| D�� | ��ӦHCl��aq��+NaOH��aq���TNaCl��aq��+H2O��l����H��0�������������������ԭ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t/min | 0 | 1 | 3 | 5 |
| n��CO��/mol | 10 | 7 | 5 | 5 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣮
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

| ʵ����� | �ζ�ǰ����/mL | �ζ������/mL |
| 1 | 0.00 | 19.96 |
| 2 | 3.26 | 23.30 |
| 3 | 1.10 | 23.40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����32.8% | B�� | С��32.8% | C�� | ����32.8% | D�� | Լ����32.8% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| t/min | 2 | 4 | 7 | 9 |
| n��H2O��/mol | 0.12 | 0.11 | 0.10 | 0.10 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com