
��18�֣�ijͬѧ���������װ��������ȡSO2����֤SO2�����ʡ�����©����װ75%��Ũ���ᣬ��ƿ��װ����Na2SO3���Իش��������⣺

����ƿ�ڷ����ķ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��ʵ������У�Ʒ����Һ����ɫ��Ϊ�� ��ʯ����Һ����ɫ�仯Ϊ�� ��ʵ��������ƿ��Ʒ����Һ����ɫ�仯Ϊ�� ��
����ˮ��SO2��Ӧ�����ӷ���ʽΪ�� ��
�ȵ��ܢٵ������ǣ� ������©���������� ��
�ɷ�����װ���ܷ����շ������� ���ش��ܡ����ܡ�����
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����NaHCO3��Na2CO3?xH2O�Ļ���Ϊ�˲ⶨxֵ��ijͬѧ���������װ�ý���ʵ�飺
����NaHCO3��Na2CO3?xH2O�Ļ���Ϊ�˲ⶨxֵ��ijͬѧ���������װ�ý���ʵ�飺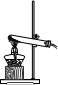

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
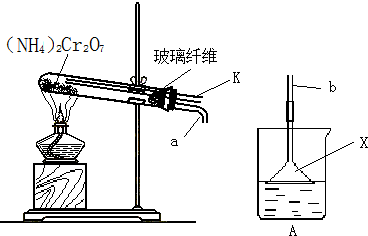
��18�֣�ijͬѧ���������װ��������ȡSO2����֤SO2�����ʡ�����©����װ75%��Ũ���ᣬ��ƿ��װ����Na2SO3���Իش��������⣺
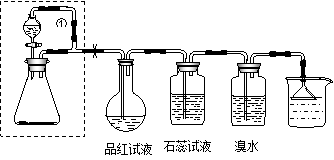
����ƿ�ڷ����ķ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��ʵ������У�Ʒ����Һ����ɫ��Ϊ�� ��ʯ����Һ����ɫ�仯Ϊ�� ��ʵ��������ƿ��Ʒ����Һ����ɫ�仯Ϊ�� ��
����ˮ��SO2��Ӧ�����ӷ���ʽΪ�� ��
�ȵ��ܢٵ������ǣ� ������©���������� ��
�ɷ�����װ���ܷ����շ������� ���ش��ܡ����ܡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ���������װ�ý���ʯ�������ʵ�飬�ش������й����⣺
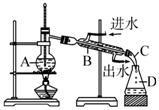
(1)ָ��ʵ��װ��������A��B��C��D�����ơ�
(2)ָ����ͬѧ����Ƶ�ʵ��װ���д��ڵĴ������������
(3)ʵ��װ�ø�������ν��������Լ�飿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�챱���и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��̽��������ⵥ�ʵ�������ǿ����ijͬѧ���������װ�ã���Ũ�����KMnO4���巴Ӧ��ȡ��������
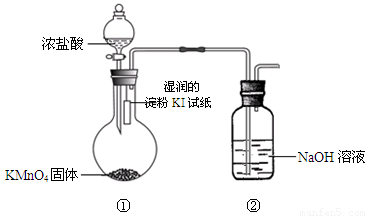
��ش�
��1��ʵ���й۲쵽ʪ��ĵ���KI��ֽ ��д�����з������û���Ӧ�����ӷ���ʽ�� ��
��2��ʵ����ۣ��ȵ��ʵķǽ����Աȵⵥ�ʵ� ���ǿ��������������ԭ�ӽṹ�ǶȽ��ͣ��Ⱥ͵�λ�����ڱ��� �壬����Ԫ�ش��ϵ��£� ���õ�������������
��3��װ�âڵ������� ��
д����Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com