
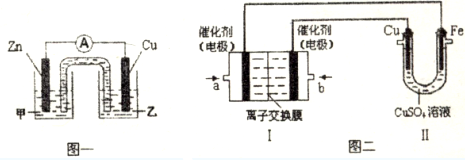
���� ��1������������������ͭ����ˮ�⣻
��2���ټ��в��ܷ�����ѧ��Ӧ�����е缫ΪZn��Ϊ��������CuΪ������ͭ���ӵõ����ӣ�
��ʵ�����϶�ͭ��CuΪ��������bΪ������aΪ����������ʧȥ���ӣ��Ѣ��е缫����Ϊ���Ե缫���缫����ͭ����Cu�����������
���2CuSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+2H2SO4��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2�����������ɵ�����Ϊ������������������ӵķŵ�˳���֪����������������ϵ����غ������㣻
��3������xCuSO4•yCu��OH��2��yCuCl2��2yHCl����x+y��CuO�����n��HCl����n��CuO��=3��2�����㣮
��� �⣺��1������������������ͭ����ˮ�⣬���ӷ�ӦΪCu2++2H2O?Cu��0H��2+2H+���ʴ�Ϊ��Cu2++2H2O?Cu��0H��2+2H+��
��2���ټ��в��ܷ�����ѧ��Ӧ�����е缫ΪZn��Ϊ�������������Һ����ZnSO4����CuΪ������ͭ���ӵõ����ӣ��缫��ӦΪCu2++2e-=Cu��
�ʴ�Ϊ��ZnSO4��Cu2++2e-=Cu��
��ʵ�����϶�ͭ��CuΪ��������bΪ������ͨ�������O2��aΪ����������ʧȥ���ӣ��缫��ӦΪCH4-8e-+10OH-=CO32-+7H2O���Ѣ��е缫����Ϊ���Ե缫���缫����ͭ����Cu�����������ᣬ��ⷴӦΪ2CuSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+2H2SO4��
�����ӵķŵ�˳���֪���������������������ɵ�����Ϊ��������������������������Ϊ672mL��n=0.03mol��
2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����
2CuSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+2H2SO4��
��������0.03mol�����壬��������������ʧ���ӣ�0.04mol������ʧȥ0.04mol���ӣ�����0.02mol�������������0.01mol����������������ʧ�������ɵ���������֪0.04mol������ʧȥ0.04mol���ӣ����ӹ�ʧȥ0.08mol��ͬʱ������ͭ���ӵõ��ӣ�0.08mol���Ӹպû�ԭ��0.04molͭ��������û�������ӵõ��ӣ�������������0.04mol���������ӣ�Ҳ����������0.04mol�����ӣ�������Ũ��Ϊ$\frac{0.04mol}{0.4L}$=0.1mol/L��pH=1��
�ʴ�Ϊ��O2��CH4-8e-+10OH-=CO32-+7H2O��2CuSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+2H2SO4��1��
��3����xCuSO4•yCu��OH��2��yCuCl2��2yHCl����x+y��CuO��
n��HCl����n��CuO��=3��2����2y����x+y��=3��2��
���x��y=1��3���ʴ�Ϊ��1��3��
���� ������Ҫ����ԭ��ؼ������غ㷨�ļ��㣬��Ŀ�ۺ��Խϴ��漰����ˮ�⡢ԭ��ء����ء����ù�ϵʽ����ȣ�ע�ضԸ߿���������Ŀ��飬��ע��֪ʶ��Ǩ�ƺ�����������ѵ������Ŀ�Ѷ��еȣ�


 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������Ǽ�����������������ﶼ�������ᷴӦ | |
| B�� | ����[KAl��SO4��2•12H2O]��ˮ�����γ�Al��OH��3���壬��������ˮ�� | |
| C�� | ClO2��������Ԫ����+4�ۣ�����ǿ�����ԣ�������Ч�ʣ���λ�������õ����ӵ���Ŀ����Cl2��5�� | |
| D�� | �������о�������������Ϊ������ʱ������Ӧ��N2+O2$\frac{\underline{\;�ŵ�\;}}{\;}$2NO��ʹ�����к���һ������NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2��SO3���Ǽ��Է��� | |
| B�� | Ԫ�ص縺��Խ���ԭ�ӣ�������������Խǿ | |
| C�� | �Ǽ��Լ����Դ����ڻ������� | |
| D�� | ���ӻ�������۵㲻һ���ȹ��ۻ�����ĸ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+һ������ | B�� | 100mL��Һ�к�0.01mol CO32- | ||
| C�� | Ba2+һ�������ڣ�Mg2+���ܴ��� | D�� | Cl-һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
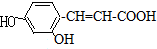 ��
��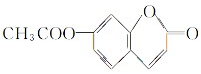 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �����Լ�A | Һ���Լ�B | |
| �� | ||
| �� | ||
| �� | ||
| �� |
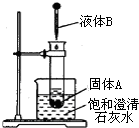
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڷŵ������£�N2��O2��ֱ�ӻ�������NO | |
| B�� | NO����������ˮ | |
| C�� | ��ʢNO�����ƿ�ǣ���������ƿ���к���ɫ�������� | |
| D�� | NO�Ǻ���ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
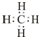 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com