
��6�֣�������A�����ϣ��������к�C 72.0%��H 6.67%������Ϊ��������������֪A����Է�������Ϊ150���ִ����������л�������ķ��ӽṹ���������ַ�����
����һ���˴Ź����Dz��A�ĺ˴Ź���������5���壬�����֮��Ϊ1��2��2��2��3��
����������������Dz��A���ӵĺ��������ͼ��ʾ��
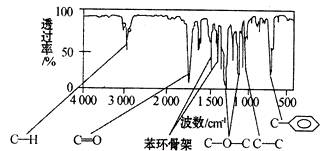
��1��A�ķ���ʽΪ________________��
��2����֪��A������ֻ��һ���������ұ�����ֻ��һ��ȡ������A����ˮ�⣬д����������������A�Ľṹ��ʽ________________��ֻдһ�֣���
��3����A������������ˮ��Ļ�ѧ����ʽΪ________________��
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������A�����ϣ��������к�C72.0%��H 6.67%�����ຬ����������������֪A����Է�������Ϊ150���ִ����������л�������ķ��ӽṹ���������ַ�����
������A�����ϣ��������к�C72.0%��H 6.67%�����ຬ����������������֪A����Է�������Ϊ150���ִ����������л�������ķ��ӽṹ���������ַ�����
 ����
���� ��
�� ��
�� ����
���� ��
�� ��
��



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com