



 ÓÅ»Æ×÷ŅµÉĻŗ£æĘ¼¼ĪÄĻ׳ö°ęÉēĻµĮŠ“š°ø
ÓÅ»Æ×÷ŅµÉĻŗ£æĘ¼¼ĪÄĻ׳ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
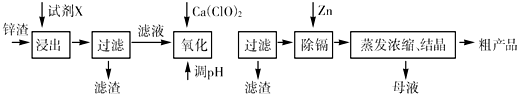
£Ø16·Ö£©Į¶Šæ³§²śÉśµÄ¹¤Ņµ·ĻŌü”Ŗ”ŖŠæŌü£Ø³żĮĖŗ¬ZnĶā£¬»¹ŗ¬ÓŠFe”¢Al”¢CdŗĶSiO2µČŌÓÖŹ£©£¬ĄūÓĆŠæŌüÖĘČ”²¢»ŲŹÕZnSO4”¤7H2OŗĶ½šŹōļÓŹĒŅ»øöÓŠŅęµÄ³¢ŹŌ£¬ĘäĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖ£ŗFe3+”¢Al3+”¢Zn2+”¢Cd2+”¢Fe2+ŅŌĒāŃõ»ÆĪļĶźČ«³ĮµķŹ±µÄpH·Ö±šĪŖ£ŗ3.2£¬4.7£¬6.5£¬9.4£¬9.7£»ŠæµÄ½šŹō»ī¶ÆŠŌ±ČļÓĒ攣
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©”°½ž³ö”±Ź±ÓƵ½µÄ”°ŹŌ¼ĮX”±ĪŖ (ĢīĆū³Ę)”£
£Ø2£©Š“³ö”°Ńõ»Æ”±¹ż³ĢµÄĄė×Ó·½³ĢŹ½ ”£
£Ø3£©”°µ÷pH”±¹ż³ĢæÉŅŌŃ”ÓĆ ”££Ø“ÓŅŌĻĀŃ”ĻīŃ”Ōń£¬ ĢīŠņŗÅ£©
A£®H2SO4 B£®ZnO C£®NaOH
”°ĀĖŌü2”±µÄÖ÷ŅŖ³É·ÖŹĒ (Ģī»ÆѧŹ½£¬ĻĀĶ¬)”£
£Ø4£©”°ŹŌ¼ĮY”±ŹĒ______________£»”°ĀĖŌü3”±µÄ³É·ÖĪŖ______________________”£
£Ø5£©”°²Ł×÷1”±µÄ·½·ØŹĒ___ ____£»ŌŚ”°²Ł×÷1”±Ź±£¬±ŲŠė²ÉČ”µÄŹµŃé“ėŹ©ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ¹ć¶«Ź”³±ÉĒĮ½ŹŠĆūŠ£øßČżÉĻѧʌʌ֊ĮŖæ¼Ąķ×ŪŹŌĢā£Ø»Æѧ²æ·Ö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©Į¶Šæ³§²śÉśµÄ¹¤Ņµ·ĻŌü”Ŗ”ŖŠæŌü£Ø³żĮĖŗ¬ZnĶā£¬»¹ŗ¬ÓŠFe”¢Al”¢CdŗĶSiO2µČŌÓÖŹ£©£¬ĄūÓĆŠæŌüÖĘČ”²¢»ŲŹÕZnSO4”¤7H2OŗĶ½šŹōļÓŹĒŅ»øöÓŠŅęµÄ³¢ŹŌ£¬ĘäĮ÷³ĢČēĻĀ£ŗ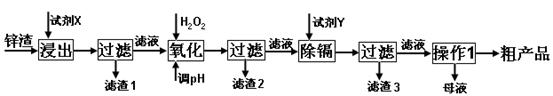
ŅŃÖŖ£ŗFe3+”¢Al3+”¢Zn2+”¢Cd2+”¢Fe2+ŅŌĒāŃõ»ÆĪļĶźČ«³ĮµķŹ±µÄpH·Ö±šĪŖ£ŗ3.2£¬4.7£¬6.5£¬9.4£¬9.7£»ŠæµÄ½šŹō»ī¶ÆŠŌ±ČļÓĒ攣
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©”°½ž³ö”±Ź±ÓƵ½µÄ”°ŹŌ¼ĮX”±ĪŖ (ĢīĆū³Ę)”£
£Ø2£©Š“³ö”°Ńõ»Æ”±¹ż³ĢµÄĄė×Ó·½³ĢŹ½ ”£
£Ø3£©”°µ÷pH”±¹ż³ĢæÉŅŌŃ”ÓĆ ”££Ø“ÓŅŌĻĀŃ”ĻīŃ”Ōń£¬ ĢīŠņŗÅ£©
A£®H2SO4 B£®ZnO C£®NaOH
”°ĀĖŌü2”±µÄÖ÷ŅŖ³É·ÖŹĒ (Ģī»ÆѧŹ½£¬ĻĀĶ¬)”£
£Ø4£©”°ŹŌ¼ĮY”±ŹĒ______________£»”°ĀĖŌü3”±µÄ³É·ÖĪŖ______________________”£
£Ø5£©”°²Ł×÷1”±µÄ·½·ØŹĒ___ ____£»ŌŚ”°²Ł×÷1”±Ź±£¬±ŲŠė²ÉČ”µÄŹµŃé“ėŹ©ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”Į¬ÖŻŹŠøßČż10ŌĀŌĀæ¼ĄķæĘ×ŪŗĻ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø16·Ö£©Į¶Šæ³§²śÉśµÄ¹¤Ņµ·ĻŌü”Ŗ”ŖŠæŌü£Ø³żĮĖŗ¬ZnĶā£¬»¹ŗ¬ÓŠFe”¢AlŗĶSiO2µČŌÓÖŹ£©£¬ĄūÓĆŠæŌüÖĘČ”²¢»ŲŹÕZnSO4”¤7H2OŗĶ½šŹōļÓŹĒŅ»øöÓŠŅęµÄ³¢ŹŌ£¬ĘäĮ÷³ĢČēĻĀ£ŗ
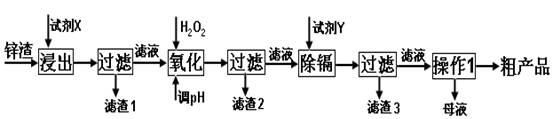
ŅŃÖŖ£ŗFe3+”¢Al3+”¢Zn2+”¢Cd2+”¢Fe2+ŅŌĒāŃõ»ÆĪļĶźČ«³ĮµķŹ±µÄpH·Ö±šĪŖ£ŗ3.2£¬4.7£¬6.5£¬9.4£¬9.7£»ŠæµÄ½šŹō»ī¶ÆŠŌ±ČļÓĒ棻SiO2ŹĒ²»ČÜÓŚĖ®ŗĶĖį£ØHF³żĶā£©µÄĖįŠŌŃõ»ÆĪļ”£
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©”°½ž³ö”±Ź±ÓƵ½µÄ”°ŹŌ¼ĮX”± Ń” ”£
A£®H2SO4(aq) B£®HCl(aq) C£®HNO3(aq)
”°ĀĖŌü1”±µÄÖ÷ŅŖ³É·ÖŹĒ _(Ģī»ÆѧŹ½)”£
£Ø2£©Š“³ö”°Ńõ»Æ”±¹ż³ĢµÄĄė×Ó·½³ĢŹ½ ”£
£Ø3£©”°µ÷pH”±¹ż³ĢæÉŅŌŃ”ÓĆ ”£
A£®H2SO4 B£®ZnO C£®NaOH
£Ø4£©”°ŹŌ¼ĮY”± Ń”______________”£
A£®Cd B£®Zn C£®Fe
£Ø5£©”°²Ł×÷1”±µÄ·½·ØŹĒ_____”¢______”¢_______”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com