
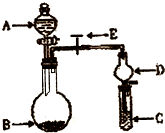
 ��ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش�
��ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
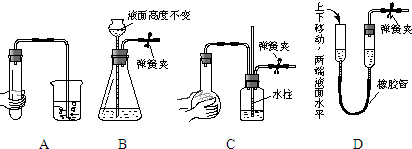
 ��2012?��ɫ��ģ��ij��ѧ��ȤС���ͬѧ����֤Mg��CO2�ķ�Ӧ������ѡ�õ�ҩƷ��Ũ���ᡢϡ���ᡢϡ���ᡢþ�ۡ�����ʯ������ʯ��ˮ������NaHCO3��Һ������Na2CO3��Һ����ѡ�õ�װ�ã�����ͼ��ʾ����Ҫʱװ�ÿ��ظ�ʹ�ã��˴����ӳ�һ��������ʵ��װ�ã�
��2012?��ɫ��ģ��ij��ѧ��ȤС���ͬѧ����֤Mg��CO2�ķ�Ӧ������ѡ�õ�ҩƷ��Ũ���ᡢϡ���ᡢϡ���ᡢþ�ۡ�����ʯ������ʯ��ˮ������NaHCO3��Һ������Na2CO3��Һ����ѡ�õ�װ�ã�����ͼ��ʾ����Ҫʱװ�ÿ��ظ�ʹ�ã��˴����ӳ�һ��������ʵ��װ�ã�| ѡ�õ�װ�� | ����ļ� | ���� |
A A |
ϡ���ᡢ����ʯ ϡ���ᡢ����ʯ |
����CO2���� ����CO2���� |
B B |
���͵�NaHCO3��Һ ���͵�NaHCO3��Һ |
��ȥ������̼�е��Ȼ������� ��ȥ������̼�е��Ȼ������� |
B B |
ŨH2SO4 ŨH2SO4 |
���������̼���� ���������̼���� |
C C |
þ�� þ�� |
þ�Ͷ�����̼��Ӧ þ�Ͷ�����̼��Ӧ |
| B | ����ʯ��ˮ | ���ն���CO2 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
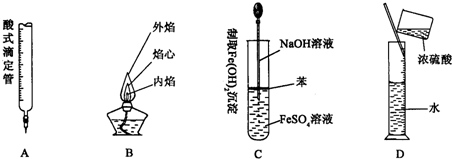
| 1 | 3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com