
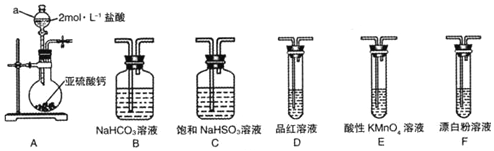
���� ��1�����������������Լ��������������ƽ��н��������ԭ��Ӧ��Ԫ�ػ��ϼ۽��͵�Ԫ�ؾ��������ԣ���������������������Ʒ�Ӧ������Ӧ�ж���������������ԣ�
��2���ٰ���A��C��F˳������װ�ã����б��͵����������Ƴ�ȥHCl����������ͨ����������Һ������������ԭ��Ӧ��
�ڴ��������ǿ�����ԡ�����������л�ԭ�ԣ��������ö���������������ֱ�ӷ�Ӧ�ж�������������������ǿ��������֤���������Ա�̼��ǿ���ٽ��̼�����Ա�HClOǿ�����жϣ�Aװ���Ʊ������������������ӷ����Ʊ��Ķ��������л���HCl���ñ��͵����������Ƴ�ȥHCl����ͨ��̼��������Һ��������֤���������Ա�̼��ǿ�������Ը��������Һ������ȥ������̼�еĶ���������Ʒ����Һ���������̼�ж��������Ƿ��������ͨ��F�У�
���ñ��͵����������Ƴ�ȥHCl��NaHSO3��Һ�����ԣ�NaHSO3��Һ�д��������������ˮ������룬ˮ�����ʼ��ԣ����뵼��������ԣ��ݴ˷���������̶ȴ���ˮ��̶ȣ�
��3���ٸ��ݹ�ϵʽ��10I-+2MnO4-+16H+=2Mn2++5I2+8H2O���������KMnO4���ٸ���5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+�����Һ��SO2�ĺ�����
�ڵζ��в�������ƿ��Һ��ҡ��������ƿ�⣬���µζ�������KMnO4�������ƫ��
��� �⣺��1��װ��A������a���л����ܿ��Ƶμ����ʵ�©���������Ƿ�Һ©����������ԭ��Ӧ��Ԫ�ػ��ϼ۽��͵�Ԫ�ؾ��������ԣ�����װ��A�в���������֤��+4�۵���Ԫ�ؾ��������ԣ���Ļ��ϼ��轵�ͣ����������������ᷴӦ������Ӧ�ж�������+4�۵���ΪΪ0�۵����������ԣ���Ӧ�ķ���ʽΪ��SO2+2H2S=3S��+2H2O�����ÿ����Ե��������������Ӧ����Ӧ�ķ���ʽΪ��SO2+2Na2S+2H2O=3S��+4NaOH��
�ʴ�Ϊ����Һ©����SO2+2H2S=3S��+2H2O�� SO2+2Na2S+2H2O=3S��+4NaOH��
��2���ٰ���A��C��F˳������װ�ã�Aװ���Ʊ���������Cװ�ã����͵����������Ƴ�ȥHCl��Fװ�ã���������ͨ����������Һ������������ԭ��Ӧ������֤��ǿ���Ʊ������ԭ��������֤��������ʹ����������ǿ����
�ʴ�Ϊ����������ͨ����������Һ������������ԭ��Ӧ������֤��ǿ���Ʊ������ԭ����
�ڴ��������ǿ�����ԡ�����������л�ԭ�ԣ��������ö���������������ֱ�ӷ�Ӧ�ж�������������������ǿ��������֤���������Ա�̼��ǿ���ٽ��̼�����Ա�HClOǿ�����жϣ�Aװ���Ʊ������������������ӷ����Ʊ��Ķ��������л���HCl���ñ��͵����������Ƴ�ȥHCl����ͨ��̼��������Һ��������֤���������Ա�̼��ǿ�������Ը��������Һ������ȥ������̼�еĶ���������Ʒ����Һ���������̼�ж��������Ƿ��������ͨ��F�У���װ������˳��ΪA��C��B��E��D��F������װ��C�������dz�ȥHCl���壬D��Ʒ�첻��ɫ��F�г��ְ�ɫ��������֤�������������ǿ�ڴ����ᣬ
�ʴ�Ϊ��B��E��D��װ��D��Ʒ����Һ����ɫ��F�г��ְ�ɫ������
��Aװ���Ʊ������������������ӷ����Ʊ��Ķ��������л���HCl���ñ��͵����������Ƴ�ȥHCl��װ��C�������dz�ȥHC1��������Ӱ������ʵ�飬NaHSO3��Һ�����ԣ�NaHSO3��Һ�д��������������ˮ������룬ˮ�����ʼ��ԣ����뵼��������ԣ��ݴ˷���������̶ȴ���ˮ��̶ȣ�������Ũ�ȴ�СΪ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
�ʴ�Ϊ����ȥHC1��������Ӱ������ʵ�飻c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��3������10I-+2MnO4-+16H+=2Mn2++5I2+8H2O���������KMnO4���ʵ���Ϊ$\frac{1}{5}$��0.200mol/L��0.015L=0.0006mol��
�������Һ��SO2��Ӧ��KMnO4���ʵ���Ϊ0.02L��0.05000mol/L-0.0006mol=0.0004mol��
��5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+�����Һ��SO2����Ϊ0.0004mol��$\frac{5}{2}$=0.001mol�����Բ�Һ��SO2�ĺ���Ϊ$\frac{0.001mol��64g/mol}{10mL��1{0}^{-3}L/mL}$=6.4g•L-1��
�ʴ�Ϊ��6.4��
��KI�������ζ�������KMnO4�����ζ��в�������ƿ��Һ��ҡ��������ƿ�⣬��������KMnO4�������ƫ�����Һ��SO2��Ӧ��KMnO4���ʵ���ƫС����Һ��SO2����ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
���� ���⿼���й�SO2���ʺͺ����ⶨʵ�飬�漰װ�÷��������ۡ�ʵ�鷽����ơ�����ʹ�á���ѧ����ȣ��ؼ�����ȷʵ��ԭ�����ϺõĿ���ѧ��ʵ���ۺ�������֪ʶǨ��Ӧ�ã���Ŀ�Ѷ��еȣ�


 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Aװ��ʵ��˵�� Ksp��AgCl����Ksp��Ag2S�� | B���Ʊ�Fe��OH��2���� | C������ȡ��ˮ�е�I2���ֳ�ˮ���IJ��� | D����¼�ζ��յ���� Ϊ12.20mL |
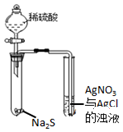 | 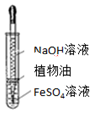 | 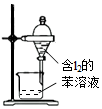 | 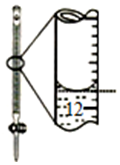 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �������ç������ijЩ�ߵ�ֲ�����е�֬��״�л� �᳣������һ����ṹ��ʽ��ͼ��ʾ������˵����ȷ���ǣ�������
�������ç������ijЩ�ߵ�ֲ�����е�֬��״�л� �᳣������һ����ṹ��ʽ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��������ç���ụΪͬ���칹�� | |
| B�� | �����Ậ�еĹ�������ȫ��ͬ | |
| C�� | ��������ܷ����ӳɷ�Ӧ���ۺϷ�Ӧ��ȡ����Ӧ | |
| D�� | �����ʵ����Ŀ������ç����ֱ�������Na��Ӧ��ͬ��ͬѹ�²���H2�������Ϊ5��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
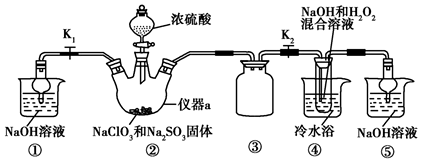
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������Ũ���H+��Ba2+��Cl-��ClO- | |
| B�� | �������NaHCO3ϡ��Һ��Na+��HCO3-��CO32-��C1O- | |
| C�� | ������� Fe��NO3��2��Һ��Ba2+��NO3-��Fe2+��C1O- | |
| D�� | ������� Na2SO4��Һ��Ba+��ClO-��Na+��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
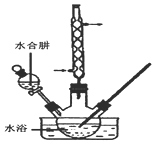 �⻯����һ�ְ�ɫ�ᾧ��ĩ��ҽ���Ͽ����ڼ�״���������μ�����̵���ȣ�ʵ������NaOH�����ʵ��ˮ����Ϊԭ���Ƶã�����װ����ͼ��ʾ��
�⻯����һ�ְ�ɫ�ᾧ��ĩ��ҽ���Ͽ����ڼ�״���������μ�����̵���ȣ�ʵ������NaOH�����ʵ��ˮ����Ϊԭ���Ƶã�����װ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����H+�ܲ�����ɫ��ζ�������Һ��OH-��K+��I-��SO32- | |
| B�� | �ڼ��������ܲ���H2����Һ�У�NH4+��Fe2+��SO42-��NO3- | |
| C�� | 0.1 mol•L-1NH4HCO3��Һ�У�K+��Na+��NO3-��Cl- | |
| D�� | �ڳ���������ɫ��Һ�У�Na+��Cu2+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ں��д���Al3+����ɫ��Һ�У�Ca2+��Na+��Cl-��HCO3- | |
| B�� | �ں��д���H+����Һ�У�Na+��K+��CO32-��NO3- | |
| C�� | ��ǿ�����Һ�У�K+��Fe3+��Cl-��NO3- | |
| D�� | �ڵμ�ʯ������ɫ����Һ�У�K+��Ca2+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com