
 Ϊ�˳��Ӧ����Դ���ù�ҵ�ϼ�Һ��Ca��OH��2��NaOH�����̵��������ճɷ�SO2������ȡʯ�ࣨCaSO4•2H2O�����������ƣ�Na2S2O8������Ƽ�Ҫ�������£�
Ϊ�˳��Ӧ����Դ���ù�ҵ�ϼ�Һ��Ca��OH��2��NaOH�����̵��������ճɷ�SO2������ȡʯ�ࣨCaSO4•2H2O�����������ƣ�Na2S2O8������Ƽ�Ҫ�������£�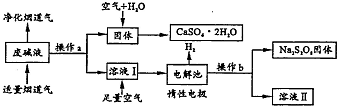
���� �̵�����S02��ϼӦ�Ļ�ѧ����ʽΪCa��OH��2+SO2=CaSO3��+H2O��2NaOH+SO2=Na2SO3+H2O������aΪ���ˣ��õ���ҺI���壻
��ҺI��Na2SO3������Ca��OH��2��NaOH��ͨ�˿���������Ӧ�Ļ�ѧ����ʽΪ2Na2SO3+O2=2Na2SO4���ö��Ե缫���ʱ��������SO42-������ʧ���ӱ���������S2O82-���缫��ӦΪ2SO42--2e-=S2O82-�������������ӷŵ������������Һ��������Ũ��������bΪ����Ũ������ȴ�ᾧ��Na2S2O8�������������˺�õ���Һ����Һ��Ϊ����Һ����ҪΪ�������ƣ���
����������е�������ˮ����������ԭ��Ӧ����CaSO4��Һ������Ũ������ȴ�ᾧ�õ�CaSO4•2H2O��
��1���̵�����S02Ϊ�����������Ӧ�����κ�ˮ������������Һ�ķ���Ϊ���ˣ�
��2������CaSO4•2H2O�����еķ�Ӧ��Ca��OH��2+SO2=CaSO3��+H2O��2CaSO3+O2=2CaSO4��CaSO4+2H2O=CaSO4•2H2O���漰���ֽ⡢������ԭ��Ӧ�����Ϸ�Ӧ�����漰�ֽ���û���Ӧ��
��3����ҺI��Na2SO3��ͨ�˿�������������ԭ��Ӧ��
��4���ö��Ե缫���ʱ��������SO42-������ʧ���ӱ���������S2O82-���缫��ӦΪ2SO42--2e-=S2O82-�������������ӷŵ������������Һ��������Ũ��������bΪ����Ũ������ȴ�ᾧ��Na2S2O8�������������˺�õ���Һ����Һ��Ϊ����Һ����ҪΪ�������ƣ�����ѭ��ʹ�ã�
��5�����������仯ʾ��ͼ�õ��ȷ�Ӧ����ʽ��
��$\frac{1}{8}$S8��s��+O2��g��+2NaOH��s��=Na2SO3��s��+H2O��l����H=-akJ/mol
��SO2��g��+2NaOH��s��=Na2SO3��s��+H2O��l����H=-bkJ/mol
���ݸ�˹���ɣ��١�8-�ڡ�8�õ�Ŀ�귴Ӧ��
��6�����ݵ缫��Ӧʽ��2SO42--2e-=S2O82-��֪��ȡa g Na2S2O8��ʧ���ӵ����ʵ���Ϊ��$\frac{ag}{238g/mol}$��2��������bL�����������������˵���������������������ŵ��������������O2��4e-������ʧ���ӵ����ʵ���Ϊ��$\frac{bL}{22.4L/mol}$��4����������ʧ���������ʵ���Ϊ��$\frac{ag}{238g/mol}$��2+$\frac{bL}{22.4L/mol}$��4����������������������õ��Ӳ�����������ϵʽΪ��H2��2e-����������ʧ���������ʵ������������õ��������ʵ����������������������
��� �⣺�̵�����S02��ϼӦ�Ļ�ѧ����ʽΪCa��OH��2+SO2=CaSO3��+H2O��2NaOH+SO2=Na2SO3+H2O������aΪ���ˣ��õ���ҺI���壻
��ҺI��Na2SO3������Ca��OH��2��NaOH��ͨ�˿���������Ӧ�Ļ�ѧ����ʽΪ2Na2SO3+O2=2Na2SO4���ö��Ե缫���ʱ��������SO42-������ʧ���ӱ���������S2O82-���缫��ӦΪ2SO42--2e-=S2O82-�������������ӷŵ������������Һ��������Ũ��������bΪ����Ũ������ȴ�ᾧ��Na2S2O8�������������˺�õ���Һ����Һ��Ϊ����Һ����ҪΪ�������ƣ���
����������е�������ˮ����������ԭ��Ӧ����CaSO4��Һ������Ũ������ȴ�ᾧ�õ�CaSO4•2H2O��
��1���̵�����S02Ϊ�����������Ӧ�����κ�ˮ���̵�����S02��ϼ����ɳ����Ļ�ѧ����ʽΪ��Ca��OH��2+SO2=CaSO3��+H2O������������Һ�ķ���Ϊ���ˣ�
�ʴ�Ϊ��Ca��OH��2+SO2=CaSO3��+H2O�����ˣ�
��2������CaSO4•2H2O�����еķ�Ӧ��Ca��OH��2+SO2=CaSO3��+H2O��2CaSO3+O2=2CaSO4��CaSO4+2H2O=CaSO4•2H2O���漰���ֽ⡢������ԭ��Ӧ�����Ϸ�Ӧ�����漰�ֽ���û���Ӧ��
�ʴ�Ϊ��CE��
��3����ҺI��Na2SO3��ͨ�˿�������������ԭ��Ӧ����ѧ����ʽΪ��2Na2SO3+O2=2Na2SO4��
�ʴ�Ϊ��2Na2SO3+O2=2Na2SO4��
��4���ö��Ե缫���ʱ��������SO42-������ʧ���ӱ���������S2O82-���缫��ӦΪ2SO42--2e-=S2O82-�������������ӷŵ������������Һ��������Ũ��������bΪ����Ũ������ȴ�ᾧ��Na2S2O8�������������˺�õ���Һ����Һ��Ϊ����Һ����ҪΪ�������ƣ�����ѭ��ʹ�ã�
�ʴ�Ϊ��2SO42--2e-=S2O82-��NaOH��
��5�����������仯ʾ��ͼ�õ��ȷ�Ӧ����ʽ��
��$\frac{1}{8}$S8��s��+O2��g��+2NaOH��s��=Na2SO3��s��+H2O��l����H=-akJ/mol
��SO2��g��+2NaOH��s��=Na2SO3��s��+H2O��l����H=-bkJ/mol
���ݸ�˹���ɣ��١�8-�ڡ�8�õ���S8��s��+8O2��g��=8SO2��g����H=-8��a-b��kJ/mol��
�ʴ�Ϊ��S8��s��+8O2��g��=8SO2��g����H=-8��a-b��kJ/mol��
��6�����ݵ缫��Ӧʽ��2SO42--2e-=S2O82-��֪��ȡa g Na2S2O8��ʧ���ӵ����ʵ���Ϊ��$\frac{ag}{238g/mol}$��2��������bL�����������������˵���������������������ŵ��������������O2��4e-������ʧ���ӵ����ʵ���Ϊ��$\frac{bL}{22.4L/mol}$��4����������ʧ���������ʵ���Ϊ��$\frac{ag}{238g/mol}$��2+$\frac{bL}{22.4L/mol}$��4����������������������õ��Ӳ�����������ϵʽΪ��H2��2e-����������ʧ���������ʵ������������õ��������ʵ��������������������������ʵ���Ϊ����$\frac{ag}{238g/mol}$��2+$\frac{bL}{22.4L/mol}$��4����2���������ɵ�H2���Ϊ����$\frac{ag}{238g/mol}$��2+$\frac{bL}{22.4L/mol}$��4����2��22.4L/mol=��$\frac{11.2a}{119}$+2b��L��
�ʴ�Ϊ��$\frac{11.2a}{119}$+2b��
���� ���⿼��ʯ�ࣨCaSO4•2H2O�����������ƣ�Na2S2O8�����Ʊ�ʵ�飬��Ҫ�漰������ԭ��Ӧ����˹���ɡ��绯ѧ��֪ʶ���ص㿼��ѧ���Թ������������˽���������������ۺ���ǿ�������߿������ض�ѧ��������������ѵ����


 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬλ�� | B�� | ͬ���칹�� | C�� | ͬ�������� | D�� | ͬ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��þƬ���� | B�� | ���������� | C�� | ���� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ṹ��ʽ | 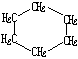 | 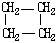 | Br-CH2-CH2-CH��CH3��-CH2-Br |
| ����ʽ | 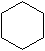 �������飩 �������飩 |  �������飩 �������飩 |  |

 ������ע����Ӧ������
������ע����Ӧ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C3H8 | B�� | C2H6 | C�� | C2H4 | D�� | C6H6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ʵ������ |
| A | ��ʢ��CH4��Cl2�ļ���ƿ���ڹ�����һ��ʱ�� | ������ɫ��dz��ƿ�ڱڸ�����״�� |
| B | ����ϩ�ڿ����е�ȼ | �������ɫ�����к��� |
| C | ��ʢ�б����Թ��м��뼸������KMnO4��Һ������� | Һ��ֲ㣬��Һ��ɫ��ȥ |
| D | ��������Һ��ϡ������ˮԡ���ȣ�ȡ��Ӧ�����Һ���������뼸�����Ƶ�Cu��OH��2����Һ���� | ����ש��ɫ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com