
���� ��1����Һ���������Ӻ�笠�����ˮ�⣬�����������ᣬ����Fe2+��NH4+����ˮ�⣻
��2��ϴ�Ӳ����ľ��巽��Ϊ���ز�������©���м�����������ˮ����û������������ˮ��Ȼ���£��ظ�2-3�Σ�ȡ���һ��ϴ��Һ������Ƿ�����������ӽ����ж��Ƿ�ϴ�Ӹɾ���
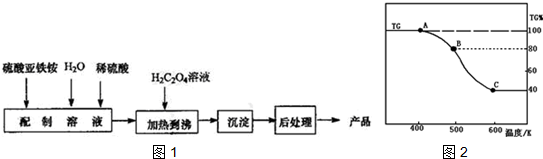
��3����B�������������ռԭ��Ʒ�������İٷ���λ80%����ʧ20%��FeC2O4.2H2O�нᾧˮ������Ϊ$\frac{36}{180}$��100%=20%����A��B������Ӧ��FeC2O4.2H2O����ʧȥ�ᾧˮ��
����ͼ��֪��������Cʱ���ֽ���ȫ���ٶ�������������Ϊ180g�������ʵ���Ϊ1mol����C���������Ϊ180g��40%=72g����Ԫ������Ϊ1mol��56g/mol=56����Ӧ������Ԫ��Ϊ72g-56g=16g������ԭ������ԭ�����ʵ���֮��Ϊ1��1��ӦΪFeO��B�㵽C����FeC2O4�ֽ�ķ�Ӧ��
��4������ʵ��ԭ����2MnO4-+5H2C2O4+6H+�T2Mn2++10CO2+8H2O��MnO4-+8H++5Fe2+�T5Fe3++Mn2++4H2O������cmol/L������ر���Һ�ζ���Ʒ��Һ����Ϊ��������������Ӷ��ܱ�������ر���Һ���������Ե�һ�����ĸ��������Һ�����ΪV1mLΪ������������������ӹ�ͬ���ģ�Ȼ��������� Zn �ۣ�ʹ��Һ�е� Fe3+ǡ��ȫ����ԭΪ Fe2+������c mol/L KMnO4��Һ�ζ�����Һ����Һ���ֵ���ɫ������KMnO4��Һ�����V2 mL�������������������ĵģ�����������������ĸ��������Һ�����Ϊ��V1-V2 ��mL���ݴ˷�����
��� �⣺��1����Һ���������Ӻ�笠�����ˮ�������������ӣ�ˮ�������ԣ������������ᣬ����Fe2+��NH4+����ˮ�⣬�ʴ�Ϊ������Fe2+��NH4+����ˮ�⣻
��2��ϴ�Ӳ����ľ��巽��Ϊ���ز�������©���м�����������ˮ����û������������ˮ��Ȼ���£��ظ�2-3�Σ������Ƿ�ϴ�Ӹɾ��ķ�����ȡ�������һ�ε�ϴ����Һ���Թ��У������еμ������ữ�� BaCl2��Һ�����ް�ɫ�����������������ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ�������һ�ε�ϴ����Һ���Թ��У������еμ������ữ�� BaCl2��Һ�����ް�ɫ�����������������ϴ�Ӹɾ���
��3������ͼ��֪����������Bʱʣ�����Ϊ80%����ʧ20%��FeC2O4.2H2O�нᾧˮ������Ϊ$\frac{36}{180}$��100%=20%����A��B������Ӧ��FeC2O4•2H2O����ʧȥ�ᾧˮ����Ӧ����ʽΪ��FeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeC2O4+2H2O��
�ʴ�Ϊ��FeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeC2O4+2H2O��
����ͼ��֪B��������Cʱ���ֽ���ȫ���ٶ�������������Ϊ180g�������ʵ���Ϊ1mol����C���������Ϊ180g��40%=72g����Ԫ������Ϊ1mol��56g/mol=56����Ӧ������Ԫ��Ϊ72g-56g=16g����ԭ�����ʵ���Ϊ1mol������ԭ������ԭ�����ʵ���֮��Ϊ1��1��ӦΪFeO������������ԭ��Ӧ�����غ�������㣬̼Ԫ�ػ��ϼ۴�+3�۱仯Ϊ+2�ۺ�+3�ۣ�����һ����̼�Ͷ�����̼���壬ԭ���غ���ƽд����ѧ����ʽ��FeC2O4 $\frac{\underline{\;\;��\;\;}}{\;}$FeO+CO��+CO2�����ʴ�Ϊ��FeC2O4 $\frac{\underline{\;\;��\;\;}}{\;}$FeO+CO��+CO2����
��4���ٲ��ᣨH2C2O4�������Ը��������Һ��Ӧ�������������ݲ�������ɫ��ʧ����Ӧ�����ӷ���ʽΪ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2+8H2O��
�ʴ�Ϊ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2+8H2O��
�ڸ������Ϸ�������ʡ�Բ��������ʹ����������ƫ�٣��������ĸ��������Һ�����ҲV2 ƫС����������������ĸ��������Һ�����Ϊ��V1-V2 ��mLƫ�����Բⶨ�IJ�������Ӻ���Ҳƫ�ʴ�Ϊ��ƫ��
�۸������Ϸ�����������������ĸ��������Һ�����Ϊ��V1-V2 ��mL����2MnO4-+5H2C2O4+6H+�T2Mn2++10CO2+8H2O������mg��Ʒ�в�������ӵ����ʵ���Ϊc��V1-V2����10-3��$\frac{5}{2}$��$\frac{100ml}{20ml}$=c��V1-V2����10-3��$\frac{25}{2}$mol���ʴ�Ϊ��c��V1-V2����10-3��$\frac{25}{2}$mol��
���� ���⿼���Ϊ�ۺϣ��漰������ʽ����д��ʵ������������ζ��ȼ��㣬ע�����ʵ��֪ʶ�Ļ��ۣ�����ʵ�鲽�衢ԭ����ע����������⣬��Ŀ�Ѷ��еȣ�


 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | ||
| C�� |  | D�� | 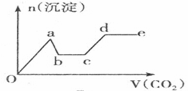 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
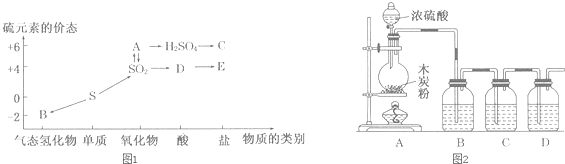
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ��Ŀ | ��� | �����Ƽ |
| A�� | ԭ�� | ʳ�Ρ���������ʯ�� | ʳ�Ρ�������������̼ |
| B�� | ���ܵĸ����� | �Ȼ��� | �Ȼ�� |
| C�� | ѭ������ | ������������̼ | �������Ȼ��� |
| D�� | ���� | ԭ���á����ʸ� | �豸���ܺĵ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| �Ҵ� | ������ | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | �����ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 1.44 | 3.1 |
| �е�/�� | 78.5 | 38.4 | 59 |
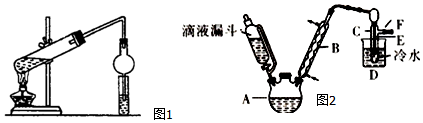
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
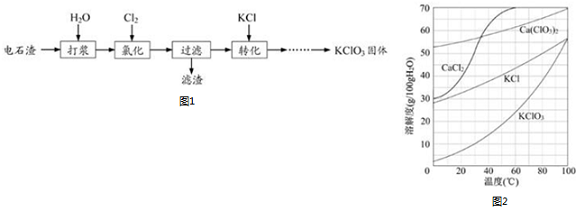
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
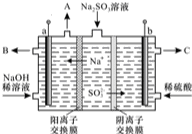 ���᳧�����ջ�����FeS2������ȡ���ᣬʵ�����������᳧��������Ҫ�ɷ���Fe2O3������FeS��SiO2���Ʊ��̷���
���᳧�����ջ�����FeS2������ȡ���ᣬʵ�����������᳧��������Ҫ�ɷ���Fe2O3������FeS��SiO2���Ʊ��̷���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
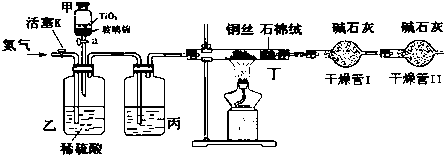
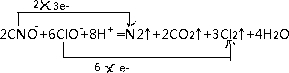 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
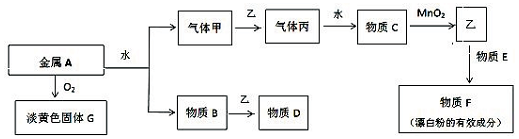
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com