
 A”¢B”¢C”¢D”¢E”¢FĮłÖÖŌŖĖŲµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬AµÄ×īøß¼ŪŗĶ×īµĶ¼ŪµÄ¾ų¶ŌÖµĻąµČ£¬BµÄ»łĢ¬Ō×ÓÓŠ3øö²»Ķ¬µÄÄܼ¶ĒŅø÷Äܼ¶ÖŠµē×ÓŹżĻąµČ£¬DµÄ»łĢ¬Ō×ÓÓėBµÄ»łĢ¬Ō×ÓĪ“³É¶Ōµē×ÓŹżÄæĻąĶ¬£¬EµÄ»łĢ¬Ō×ÓsÄܼ¶µÄµē×Ó×ÜŹżÓėpÄܼ¶µÄµē×ÓŹżĻąµČ£¬FµÄ»łĢ¬Ō×ÓµÄ3d¹ģµĄŹĒ4sµē×ÓŹżµÄ4±¶£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
A”¢B”¢C”¢D”¢E”¢FĮłÖÖŌŖĖŲµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬AµÄ×īøß¼ŪŗĶ×īµĶ¼ŪµÄ¾ų¶ŌÖµĻąµČ£¬BµÄ»łĢ¬Ō×ÓÓŠ3øö²»Ķ¬µÄÄܼ¶ĒŅø÷Äܼ¶ÖŠµē×ÓŹżĻąµČ£¬DµÄ»łĢ¬Ō×ÓÓėBµÄ»łĢ¬Ō×ÓĪ“³É¶Ōµē×ÓŹżÄæĻąĶ¬£¬EµÄ»łĢ¬Ō×ÓsÄܼ¶µÄµē×Ó×ÜŹżÓėpÄܼ¶µÄµē×ÓŹżĻąµČ£¬FµÄ»łĢ¬Ō×ÓµÄ3d¹ģµĄŹĒ4sµē×ÓŹżµÄ4±¶£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö A”¢B”¢C”¢D”¢E”¢FĮłÖÖŌŖĖŲµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£®ĘäÖŠBµÄ»łĢ¬Ō×ÓÓŠ3øö²»Ķ¬µÄÄܼ¶£¬ĒŅø÷Äܼ¶ÖŠµÄµē×ÓŹżĻąµČ£¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p2£¬¹ŹBĪŖĢ¼ŌŖĖŲ£»AµÄ×īøßÕż¼ŪŗĶ×īµĶøŗ¼ŪµÄ¾ų¶ŌÖµĻąµČ£¬Ō×ÓŠņŹż“óÓŚĢ¼£¬¹ŹAĪŖHŌŖĖŲ£»EµÄ»łĢ¬Ō×ÓµÄsÄܼ¶µÄµē×Ó×ÜŹżÓėpÄܼ¶µÄµē×ÓŹżĻąµČ£¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p4»ņ1s22s22p63s2£¬DµÄ»łĢ¬Ō×ÓÓėBµÄ»łĢ¬Ō×ÓµÄĪ“³É¶Ōµē×ÓŹżÄæĻąĶ¬£¬DµÄŌ×ÓŠņŹżŠ”ÓŚE£¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ö»ÄÜĪŖ1s22s22p4£¬ŌņDĪŖOŌŖĖŲ£¬¹ŹEĪŖMg£¬¶ųCµÄŌ×ÓŠņŹż½éÓŚĢ¼”¢ŃõÖ®¼ä£¬ŌņCĪŖNŌŖĖŲ£»FµÄ»łĢ¬Ō×ÓµÄ3d¹ģµĄµē×ÓŹżŹĒ4sµē×ÓŹżµÄ4±¶£¬4sµē×ÓÖ»ÄÜÓŠ2øöµē×Ó²ć£¬¹ŹŌ×ÓŗĖĶāµē×ÓÅŲ¼ĪŖ1s22s22p63s23p63d84s2£¬¹ŹFĪŖNi£¬ŅŌ“ĖĄ“½ā“šøĆĢā£®
½ā“š ½ā£ŗA”¢B”¢C”¢D”¢E”¢FĮłÖÖŌŖĖŲµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£®ĘäÖŠBµÄ»łĢ¬Ō×ÓÓŠ3øö²»Ķ¬µÄÄܼ¶£¬ĒŅø÷Äܼ¶ÖŠµÄµē×ÓŹżĻąµČ£¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p2£¬¹ŹBĪŖĢ¼ŌŖĖŲ£»AµÄ×īøßÕż¼ŪŗĶ×īµĶøŗ¼ŪµÄ¾ų¶ŌÖµĻąµČ£¬Ō×ÓŠņŹż“óÓŚĢ¼£¬¹ŹAĪŖHŌŖĖŲ£»EµÄ»łĢ¬Ō×ÓµÄsÄܼ¶µÄµē×Ó×ÜŹżÓėpÄܼ¶µÄµē×ÓŹżĻąµČ£¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p4»ņ1s22s22p63s2£¬DµÄ»łĢ¬Ō×ÓÓėBµÄ»łĢ¬Ō×ÓµÄĪ“³É¶Ōµē×ÓŹżÄæĻąĶ¬£¬DµÄŌ×ÓŠņŹżŠ”ÓŚE£¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ö»ÄÜĪŖ1s22s22p4£¬ŌņDĪŖOŌŖĖŲ£¬¹ŹEĪŖMg£¬¶ųCµÄŌ×ÓŠņŹż½éÓŚĢ¼”¢ŃõÖ®¼ä£¬ŌņCĪŖNŌŖĖŲ£»FµÄ»łĢ¬Ō×ÓµÄ3d¹ģµĄµē×ÓŹżŹĒ4sµē×ÓŹżµÄ4±¶£¬4sµē×ÓÖ»ÄÜÓŠ2øöµē×Ó²ć£¬¹ŹŌ×ÓŗĖĶāµē×ÓÅŲ¼ĪŖ1s22s22p63s23p63d84s2£¬¹ŹFĪŖNi£¬
£Ø1£©FµÄ»łĢ¬Ō×ÓµÄ3d¹ģµĄµē×ÓŹżŹĒ4sµē×ÓŹżµÄ4±¶£¬4sµē×ÓÖ»ÄÜÓŠ2øöµē×Ó²ć£¬3dÄܼ¶ÓŠ8øöµē×Ó£¬¹ŹŌ×ÓŗĖĶāµē×ÓÅŲ¼ĪŖ1s22s22p63s23p63d84s2£¬¼Ūµē×ÓÅŲ¼ĪŖ3d84s2£¬
¹Ź“š°øĪŖ£ŗ3d84s2£»
£Ø2£©C”¢N”¢O”¢MgĖÄÖÖŌŖĖŲ£¬MgŹōÓŚ½šŹō£¬µŚŅ»µēĄėÄÜ×īŠ”£¬C”¢N”¢OĶ¬ÖÜĘŚ£¬ĖęŌ×ÓŠņŹżŌö“óµÄµŚŅ»µēĄėÄܳŹŌö“óĒ÷ŹĘ£¬µ«NŌŖĖŲ2pÄܼ¶ČŻÄÉ3øöµē×Ó£¬“¦ÓŚ°ėĀśĪȶØדĢ¬£¬ÄÜĮæ½ĻµĶ£¬µŚŅ»µēĄėÄÜøßÓŚĶ¬ÖÜĘŚĻąĮŚŌŖĖŲ£¬¹ŹµŚŅ»µēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņĪŖMg£¼C£¼O£¼N£¬
¹Ź“š°øĪŖ£ŗMg£¼C£¼O£¼N£»
£Ø3£©A£®C2H2·Ö×ÓÖŠŗ¬ÓŠC”ŌCČż¼ü”¢C-H¼ü£¬ŗ¬ÓŠ¦Ņ¼üŗĶ¦Š¼ü£¬¶ųH2O2·Ö×ÓĪŖH-O-O-H£¬Ö»ŗ¬ÓŠ¦Ņ¼ü£¬¹ŹA“ķĪó£»
B£®C2H2·Ö×ÓÖŠCŌ×Ó³É2øö¦Ņ¼ü”¢Ć»ÓŠ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæĪŖ2£¬²ÉČ”spŌӻƣ¬H2O2·Ö×ÓÖŠOŌ×Ó³É2øö¦Ņ¼ü”¢ŗ¬ÓŠ2¶Ō¹Āµē×Ó¶Ō£¬ŌӻƹģµĄŹżÄæĪŖ4£¬²ÉČ”sp3Ōӻƣ¬¹ŹB“ķĪó£»
C£®C2H2ŹĒÖ±ĻߊĶ¶Ō³Ę½į¹¹£¬ŹĒ·Ē¼«ŠŌ·Ö×Ó£¬¶ųH2O2·Ö×ÓŹĒÕ¹æŖŹéŅ³ŠĪ½į¹¹£¬ŹōÓŚ¼«ŠŌ·Ö×Ó£¬¹ŹC“ķĪó£»
D£®C2H2·Ö×Ó”¢H2O2·Ö×Óŗ¬ÓŠµē×Ó×ÜŹż»ņ¼Ūµē×Ó×ÜŹż²»ĻąµČ£¬²»ŹĒµČµē×ÓĢ壬¹ŹD“ķĪó£»
E£®C2H2³£ĪĀĻĀĪŖĘųĢ¬£¬¶ųH2O2³£ĪĀĻĀĪŖŅŗĢ¬£¬¹ŹEÕżČ·£¬
¹Ź“š°øĪŖ£ŗE£»
£Ø4£©Ni2+ÄÜÓėCO·Ö×ÓĶعżÅäĪ»¼üŠĪ³É[Ni£ØCO£©4]2+£¬ĘäŌŅņŹĒCO·Ö×ÓÖŠŗ¬ÓŠ¹Āµē×Ó¶Ō£¬¹Ź“š°øĪŖ£ŗ¹Āµē×Ó¶Ō£»
£Ø5£©ŅŌÉĻµ×ĆęĆęŠÄFŌ×ÓŃŠ¾æ£¬ÓėÖ®¾ąĄė×ī½üµÄFŌ×ÓĪ»ÓŚ¾§°ūĮ½øö²ąĆę¼°Ē°ŗóĆęĆęŠÄÉĻ£¬¶ų¾§°ūÉĻµ×ĆęĪŖÉĻ²ć¾§°ūµÄĻĀµ×Ćę£¬¹ŹÓėÖ®×ī½üµÄFŌ×ÓÓŠ8øö£»
Óɾ§°ū¾§°ūæÉÖŖ£¬¾§°ūÖŠCŌ×ÓŹżÄæ=1”¢NiŌŖĖŲŹżÄæ=6”Į$\frac{1}{2}$=3”¢MgŌ×ÓŹżÄæ=8”Į$\frac{1}{8}$=1£¬¹ŹøĆ¾§ĢåµÄ»ÆѧŹ½ĪŖMgNi3C£»
¾§°ūÖŹĮæ=$\frac{M}{{N}_{A}}$g£¬ÉĻµ×Ćę¶Ō½ĒĻß³¤¶Č=£Ø2r2+2r3£©pm=£Ø2r2+2r3£©”Į10-10cm£¬¹Ź¾§°ūĄā³¤=$\frac{\sqrt{2}}{2}$”Į£Ø2r2+2r3£©”Į10-10cm=$\sqrt{2}$£Ør2+r3£©”Į10-10cm£¬¾§°ūĢå»ż=[$\sqrt{2}$£Ør2+r3£©”Į10-10cm]3=2$\sqrt{2}$£Ør2+r3£©3”Į10-30cm3£¬¹Ź¾§°ūĆܶČ=$\frac{\frac{M}{{N}_{A}}\;g}{2\sqrt{2}£Ø{r}_{2}+{r}_{3}£©^{3}”Į1{0}^{-30}\;c{m}^{3}}$=$\frac{1{0}^{30}M}{2\sqrt{2}£Ø{r}_{2}+{r}_{3}£©^{3}•{N}_{A}}$g£®cm-3£¬
¹Ź“š°øĪŖ£ŗ8£»MgNi3C£»$\frac{1{0}^{30}M}{2\sqrt{2}£Ø{r}_{2}+{r}_{3}£©^{3}•{N}_{A}}$£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹ½į¹¹ÓėŠŌÖŹ£¬Éę¼°ŗĖĶāµē×ÓÅŲ¼”¢µēĄėÄÜ”¢·Ö×Ó½į¹¹ÓėŠŌÖŹ”¢ÅäŗĻĪļ”¢¾§°ū½į¹¹Óė¼ĘĖćµČ£¬Ń§ÉśŅŖŹģĮ·ÕĘĪÕ»ł“”ÖŖŹ¶£¬²¢ÄÜĒØŅĘŌĖÓĆ£¬£Ø5£©ÖŠ¾§°ū½į¹¹Óė¼ĘĖć£¬ĪŖŅדķµć£¬ŠčŅŖѧɜ¾ßÓŠŅ»¶ØµÄæÕ¼äĻėĻóÓėŹżŃ§¼ĘĖćÄÜĮ¦£¬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | £Ø3£©£Ø4£©£Ø5£© | B£® | £Ø4£©£Ø5£©£Ø7£© | C£® | £Ø4£©£Ø7£© | D£® | £Ø3£©£Ø4£©£Ø5£©£Ø7£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | SiO2¾§Ģå½į¹¹ÖŠ£¬ĆæøöSiŌ×ÓÓė2øöOŌ×ÓÖ±½ÓĻąĮ¬ | |
| B£® | Ķس£×“æöĻĀ£¬60gSiO2¾§ĢåÖŠŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA£ØNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£© | |
| C£® | 12g½šøÕŹÆŗ¬4molC-C¼ü | |
| D£® | ½šøÕŹÆĶųד½į¹¹ÖŠ£¬Óɹ²¼Ū¼üŠĪ³ÉµÄĢ¼Ō×Ó»·ÖŠ£¬×īŠ”µÄ»·ÉĻÓŠ6øöĢ¼Ō×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
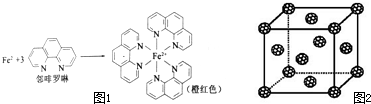
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
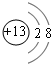 Ӣ
”¢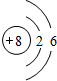 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ī¢Į£°ė¾¶Na+£¼K+£¼Cl-£¼S2- | B£® | ĪČ¶ØŠŌHI£¾HBr£¾HCl£¾HF | ||
| C£® | ĖįŠŌH2SiO3£¼H3PO4£¼H2SO4£¼HClO4 | D£® | ¼īŠŌKOH£¾Ca£ØOH£©2£¾Mg£ØOH£©2£¾Al£ØOH£©3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | b=a+34 | B£® | a=b-11 | C£® | b=a+25 | D£® | a=b-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ŹµŃéĢ½¾æŹĒѧĻ°»ÆѧµÄŅ»øöÖŲŅŖ·½·Ø£¬Ä³ŹµŃ銔×éµÄĶ¬Ń§ĄūÓĆĻĀĮŠ×°ÖĆĶź³ÉŅ»Š©³£¼ūĘųĢåÖʱøŅŌ¼°Ļą¹ŲĪļÖŹŠŌÖŹĢ½¾æ£Ø¼Š³Ö×°ÖĆ¼°Į¬½ÓĻš½ŗ¹ÜŅŃŹ”ĀŌ£¬Ęä֊װÖĆEÓŠ¶ąøö¹©Ź¹ÓĆ£©£®
ŹµŃéĢ½¾æŹĒѧĻ°»ÆѧµÄŅ»øöÖŲŅŖ·½·Ø£¬Ä³ŹµŃ銔×éµÄĶ¬Ń§ĄūÓĆĻĀĮŠ×°ÖĆĶź³ÉŅ»Š©³£¼ūĘųĢåÖʱøŅŌ¼°Ļą¹ŲĪļÖŹŠŌÖŹĢ½¾æ£Ø¼Š³Ö×°ÖĆ¼°Į¬½ÓĻš½ŗ¹ÜŅŃŹ”ĀŌ£¬Ęä֊װÖĆEÓŠ¶ąøö¹©Ź¹ÓĆ£©£®| ŅŗĢåŹŌ¼Į | ¹ĢĢåŅ©Ę· |
| Ļ”ĮņĖį”¢Ļ”ŃĪĖį”¢Ļ”ĻõĖį”¢NaOHČÜŅŗ”¢ÅØ°±Ė®”¢5%H2O2ČÜŅŗ”¢ÅØŃĪĖį”¢±„ŗĶŹ³ŃĪĖ® | CaCO3”¢CaO”¢MnO2”¢KMnO4”¢ ¼īŹÆ»Ņ”¢Cu”¢Zn”¢Na2S |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com