
 ʵ��������480mL 0.1mol?L-1NaOH��Һ���ش���������
ʵ��������480mL 0.1mol?L-1NaOH��Һ���ش���������| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������������˪Ϊ1.98mg |
| B���ֽ����������Ϊ0.672mL |
| C������˪��Ӧ��пΪ3.90mg |
| D��ת�Ƶĵ�������Ϊ6��10-5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Cl2 |
| B��CO2 |
| C��CH4 |
| D��O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
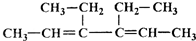

 R��R�����������
R��R������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ʵ�鲽�� | ����ʵ����� ���������������װʵ��װ�ã� |
| �� | �����η����ձ��У�������ʱ������ˮ����ֽ��裬ֱ������ȫ����ʧ | |
| �� | ����NaOH��Һ | ��μ�������������Һ��ֱ�����ٳ��ֳ���Ϊֹ |
| �� | ���� |
��μ������Һ��ֱ�����ٳ��ֳ���Ϊֹ |
| �� | ���� |
��μ������Һ��ֱ�����ٳ��ֳ���Ϊֹ |
| �� | ���� | ��װ�ù������������ձ��е�����Һ�ز���������������й��� |
| �� | �μ����� | ����Һ����μ������ᣬ����pH��ֽ�����Һ������Һ������ |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ��
��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com