
£Øl£©ĀĮÓėijŠ©½šŹōŃõ»ÆĪļŌŚøßĪĀĻĀµÄ·“Ó¦³ĘĪŖĀĮČČ·“Ó¦£¬æÉÓĆÓŚŅ±Į¶øßČŪµć½šŹō”£
ŅŃÖŖ£ŗ4Al£Øs£©£«3O2£Øg£©=2Al2O3£Øs£© 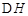 £½-2830kJ”¤mol-1
£½-2830kJ”¤mol-1
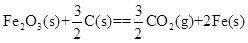
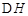 =+230kJ”¤mol-1
=+230kJ”¤mol-1
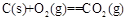
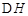 =-390kJ”¤mol-1
=-390kJ”¤mol-1
ĀĮÓėŃõ»ÆĢś·¢ÉśĀĮČČ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø2£©ČēĻĀĶ¼ĖłŹ¾£¬ø÷ČŻĘ÷ÖŠŹ¢ÓŠŗ£Ė®£¬ĢśŌŚĘäÖŠ±»øÆŹ“Ź±ÓÉæģµ½ĀżµÄĖ³ŠņŹĒ £»
¢Ś×°ÖĆÖŠCuµē¼«ÉĻµÄµē¼«·“Ó¦Ź½ĪŖ ”£
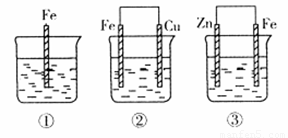
£Ø3£©·°£ØV£©¼°Ęä»ÆŗĻĪļ¹ć·ŗÓ¦ÓĆÓŚŠĀÄÜŌ“ĮģÓņ”£Č«·°ŅŗĮ÷“¢Äܵē³ŲŹĒĄūÓĆ²»Ķ¬¼ŪĢ¬Ąė×Ó¶ŌµÄŃõ»Æ»¹Ō·“Ó¦Ą“ŹµĻÖ»ÆѧÄÜŗĶµēÄÜĻą»„×Ŗ»ÆµÄ×°ÖĆ£¬ĘäŌĄķČēĶ¼ĖłŹ¾”£
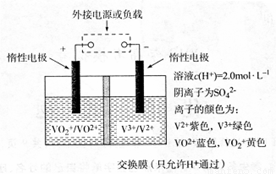
¢Łµ±×ó²ŪČÜŅŗÖš½„Óɻʱ䥶£¬Ęäµē¼«·“Ó¦Ź½ĪŖ ”£
¢Ś³äµē¹ż³ĢÖŠ£¬ÓŅ²ŪČÜŅŗŃÕÉ«Öš½„ÓÉ É«±äĪŖ É«”£
£Ø9·Ö£©
£Ø1£©2Al(s)+Fe2O3(s)= Al 2O3(s)+ 2Fe(s) ”÷H=-600kJ”¤mol(2·Ö)
£Ø2£©¢Ś>¢Ł>¢Ū O2+2H2O+4e-=4OH-
£Ø3£©¢ŁVO2-+2H++e-= VO2++ H2O(2·Ö)£»¢ŚĀĢ(1·Ö)£»×Ļ(1·Ö)
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ”£
£Ø1£©·Ö±š±ź¼ĒČżøö·½³ĢŹ½ĪŖ¢ŁŹ½”¢¢ŚŹ½”¢¢ŪŹ½£¬øł¾ŻøĒĖ¹¶ØĀÉ¢ŁŹ½-¢ŚŹ½”Į2-¢ŪŹ½”Į3µĆ2Al(s)+Fe2O3(s)= Al 2O3(s)+ 2Fe(s) ”÷H=-600kJ”¤mol ”£
£Ø2£©øÆŹ“ĖŁĀŹ£ŗµē»ÆѧøÆŹ“>»ÆѧøÆŹ“>±»±£»¤µÄøÆŹ“£¬ĖłŅŌ¢Ś>¢Ł>¢Ū£»¢Ś×°ÖĆÖŠCuµē¼«ĪŖÕż¼«£¬O2·Åµē£¬O2+2H2O+4e-=4OH-”£
£Ø3£©¹Ū²ģĶ¼Ę¬£¬¢ŁVO2-£Ø»ĘÉ«£©”¢ VO2+£ØĄ¶É«£©£¬Ęäµē¼«·“Ó¦Ź½ĪŖVO2-+2H++e-= VO2++ H2O”£¢Ś³äµē¹ż³ĢÖŠ£¬ÓŅ²ŪµÄµē×Ó£¬¼ŪĢ¬½µµĶ”£
æ¼µć£ŗ±¾ĢāøĒĖ¹¶ØĀÉ”¢æ¼²éµē³Ų”¢µē½ā³ŲµČĻą¹ŲÖŖŹ¶”£


 ÓżӾ«¾ķĻµĮŠ“š°ø
ÓżӾ«¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģø£½ØŹ”ĖĵŲĮłŠ£øßČżµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĢśŗĶĀĮŹĒĮ½ÖÖÖŲŅŖµÄ½šŹō£¬ĖüĆĒµÄµ„ÖŹ¼°»ÆŗĻĪļÓŠ×Åø÷×ŌµÄŠŌÖŹ”£
(1)ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬Ńõ»ÆŃĒĢśæÉŅŌÓėŅ»Ńõ»ÆĢ¼·¢ÉśĻĀĮŠ·“Ó¦£ŗ
Fe2O3£Øs£©£«3CO£Øg£© 2Fe£Øs£©£«3CO2£Øg£©¦¤H£¾0
2Fe£Øs£©£«3CO2£Øg£©¦¤H£¾0
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ£ŗK=
¢ŚøĆĪĀ¶ČĻĀ£¬ŌŚ2LŹ¢ÓŠ ·ŪÄ©µÄĆܱÕČŻĘ÷ÖŠĶØČėCOĘųĢ壬10minŗó£¬Éś³ÉĮĖµ„ÖŹĢś11£®2g”£Ōņ10minÄŚCOµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ
·ŪÄ©µÄĆܱÕČŻĘ÷ÖŠĶØČėCOĘųĢ壬10minŗó£¬Éś³ÉĮĖµ„ÖŹĢś11£®2g”£Ōņ10minÄŚCOµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ
(2)ijŠ©½šŹōŃõ»ÆĪļ·ŪÄ©ŗĶAl·ŪŌŚĆ¾ĢõµÄŅżČ¼ĻĀæÉŅŌ·¢ÉśĀĮČČ·“Ó¦”£ĻĀĮŠ·“Ó¦ĖŁĀŹ(v)
ŗĶĪĀ¶Č(T)µÄ¹ŲĻµŹ¾ŅāĶ¼ÖŠÓėĀĮČČ·“Ó¦×ī½Ó½üµÄŹĒ ”£
(3)Fe3+ŃĪŗĶAl3+ŃĪŌŚŠŌÖŹÉĻÓŠŗܶąĻąĖʵĵŲ·½£¬ČēÓö¼ī¶¼Éś³ÉÄŃČܵĽŗד³Įµķ£¬æÉÓĆÓŚ¾»Ė®£»Ņ²ÓŠ²»Ķ¬Ö®“¦£¬ČēFe3+¼ČÓŠŃõ»ÆŠŌÓÖÓŠ»¹ŌŠŌ£¬Al3+Ö»ÓŠŃõ»ÆŠŌ”£µ«Fe3+Ö»ÓŠŌŚ¼īŠŌ½éÖŹÖŠ²ÅÄܱ»Ńõ»ÆĪŖFeO42-£¬ĒėĶź³ÉĻĀĮŠ·½³ĢŹ½£ŗ
Fe(OH)3 + ClO- + == FeO42- + Cl- + ;
(4)Čō½«ag FeŗĶAlµÄ»ģŗĻĪļČÜÓŚ2mol/LµÄ×ćĮæµÄĮņĖįÖŠ£¬ŌŁĶłČÜŅŗÖŠ¼ÓČė×ćĮæµÄ6mol/LµÄNaOHČÜŅŗ£¬³ä·Ö·“Ó¦£¬¹żĀĖ£¬Ļ“µÓ£¬øÉŌļ×ĘÉÕ£¬³ĘĮæĖłµĆ¹ĢĢåµÄÖŹĮæČŌĪŖag£¬ŌņŌ»ģŗĻĪļÖŠAlµÄÖŹĮæ·ÖŹżĪŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģø£½ØŹ”ČżĆ÷¾ÅÖŠøßČżÉĻѧʌµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĢśŗĶĀĮŹĒĮ½ÖÖÖŲŅŖµÄ½šŹō£¬ĖüĆĒµÄµ„ÖŹ¼°»ÆŗĻĪļÓŠ×Åø÷×ŌµÄŠŌÖŹ”£
(1)ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬Ńõ»ÆĢśæÉŅŌÓėŅ»Ńõ»ÆĢ¼·¢ÉśĻĀĮŠ·“Ó¦£ŗ
Fe2O3£Øs£©£«3CO£Øg£© 2Fe£Øs£©£«3CO2£Øg£©¦¤H£¾0
2Fe£Øs£©£«3CO2£Øg£©¦¤H£¾0
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ£ŗK=
¢ŚøĆĪĀ¶ČĻĀ£¬ŌŚ2LŹ¢ÓŠ ·ŪÄ©µÄĆܱÕČŻĘ÷ÖŠĶØČėCOĘųĢ壬10minŗó£¬Éś³ÉĮĖµ„ÖŹĢś11.2g”£Ōņ10minÄŚCOµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ
·ŪÄ©µÄĆܱÕČŻĘ÷ÖŠĶØČėCOĘųĢ壬10minŗó£¬Éś³ÉĮĖµ„ÖŹĢś11.2g”£Ōņ10minÄŚCOµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ
(2)ijŠ©½šŹōŃõ»ÆĪļ·ŪÄ©ŗĶAl·ŪŌŚĆ¾ĢõµÄŅżČ¼ĻĀæÉŅŌ·¢ÉśĀĮČČ·“Ó¦”£ĻĀĮŠ·“Ó¦ĖŁĀŹ(v)ŗĶĪĀ¶Č(T)µÄ¹ŲĻµŹ¾ŅāĶ¼ÖŠÓėĀĮČČ·“Ó¦×ī½Ó½üµÄŹĒ ”£
(3)Fe3+ŃĪŗĶAl3+ŃĪŌŚŠŌÖŹÉĻÓŠŗܶąĻąĖʵĵŲ·½£¬ČēÓö¼ī¶¼Éś³ÉÄŃČܵĽŗד³Įµķ£¬æÉÓĆÓŚ¾»Ė®£»Ņ²ÓŠ²»Ķ¬Ö®“¦£¬ČēFe3+¼ČÓŠŃõ»ÆŠŌÓÖÓŠ»¹ŌŠŌ£¬Al3+Ö»ÓŠŃõ»ÆŠŌ”£µ«Fe3+Ö»ÓŠŌŚ¼īŠŌ½éÖŹÖŠ²ÅÄܱ»Ńõ»ÆĪŖFeO42-£¬ĒėĶź³ÉĻĀĮŠ·½³ĢŹ½£ŗ
Fe(OH)3 + ClO- + == FeO42- + Cl- + ;
(4)Čō½«ag FeŗĶAlµÄ»ģŗĻĪļČÜÓŚ2mol/LµÄ×ćĮæµÄĮņĖįÖŠ£¬ŌŁĶłČÜŅŗÖŠ¼ÓČė×ćĮæµÄ6mol/LµÄNaOHČÜŅŗ£¬³ä·Ö·“Ó¦£¬¹żĀĖ£¬Ļ“µÓ£¬øÉŌļ×ĘÉÕ£¬³ĘĮæĖłµĆ¹ĢĢåµÄÖŹĮæČŌĪŖag£¬ŌņŌ»ģŗĻĪļÖŠAlµÄÖŹĮæ·ÖŹżĪŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğø£½ØŹ”øßČżÉĻѧʌµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĢśŗĶĀĮŹĒĮ½ÖÖÖŲŅŖµÄ½šŹō£¬ĖüĆĒµÄµ„ÖŹ¼°»ÆŗĻĪļÓŠ×Åø÷×ŌµÄŠŌÖŹ”£
(1)ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬Ńõ»ÆĢśæÉŅŌÓėŅ»Ńõ»ÆĢ¼·¢ÉśĻĀĮŠ·“Ó¦£ŗ
Fe2O3£Øs£©£«3CO£Øg£© 2Fe£Øs£©£«3CO2£Øg£©¦¤H£¾0
2Fe£Øs£©£«3CO2£Øg£©¦¤H£¾0
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ£ŗK=
¢ŚøĆĪĀ¶ČĻĀ£¬ŌŚ2LŹ¢ÓŠ ·ŪÄ©µÄĆܱÕČŻĘ÷ÖŠĶØČėCOĘųĢ壬10minŗó£¬Éś³ÉĮĖµ„ÖŹĢś11.2g”£Ōņ10minÄŚCOµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ
·ŪÄ©µÄĆܱÕČŻĘ÷ÖŠĶØČėCOĘųĢ壬10minŗó£¬Éś³ÉĮĖµ„ÖŹĢś11.2g”£Ōņ10minÄŚCOµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ
(2)ijŠ©½šŹōŃõ»ÆĪļ·ŪÄ©ŗĶAl·ŪŌŚĆ¾ĢõµÄŅżČ¼ĻĀæÉŅŌ·¢ÉśĀĮČČ·“Ó¦”£ĻĀĮŠ·“Ó¦ĖŁĀŹ(v)ŗĶĪĀ¶Č(T)µÄ¹ŲĻµŹ¾ŅāĶ¼ÖŠÓėĀĮČČ·“Ó¦×ī½Ó½üµÄŹĒ ”£

(3)Fe3+ŃĪŗĶAl3+ŃĪŌŚŠŌÖŹÉĻÓŠŗܶąĻąĖʵĵŲ·½£¬ČēÓö¼ī¶¼Éś³ÉÄŃČܵĽŗד³Įµķ£¬æÉÓĆÓŚ¾»Ė®£»Ņ²ÓŠ²»Ķ¬Ö®“¦£¬ČēFe3+¼ČÓŠŃõ»ÆŠŌÓÖÓŠ»¹ŌŠŌ£¬Al3+Ö»ÓŠŃõ»ÆŠŌ”£µ«Fe3+Ö»ÓŠŌŚ¼īŠŌ½éÖŹÖŠ²ÅÄܱ»Ńõ»ÆĪŖFeO42-£¬ĒėĶź³ÉĻĀĮŠ·½³ĢŹ½£ŗ
Fe(OH)3 + ClO- + == FeO42- + Cl- + ;
(4)Čō½«ag FeŗĶAlµÄ»ģŗĻĪļČÜÓŚ2mol/LµÄ×ćĮæµÄĮņĖįÖŠ£¬ŌŁĶłČÜŅŗÖŠ¼ÓČė×ćĮæµÄ6mol/LµÄNaOHČÜŅŗ£¬³ä·Ö·“Ó¦£¬¹żĀĖ£¬Ļ“µÓ£¬øÉŌļ×ĘÉÕ£¬³ĘĮæĖłµĆ¹ĢĢåµÄÖŹĮæČŌĪŖag£¬ŌņŌ»ģŗĻĪļÖŠAlµÄÖŹĮæ·ÖŹżĪŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğĢģ½ņŹŠøßČżŗ®¼ŁŃéŹÕæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©ĢśŗĶĀĮŹĒĮ½ÖÖÖŲŅŖµÄ½šŹō£¬ĖüĆĒµÄµ„ÖŹ¼°»ÆŗĻĪļÓŠ×Åø÷×ŌµÄŠŌÖŹ”£
£Ø1£©ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬Ńõ»ÆĢśæÉŅŌÓėŅ»Ńõ»ÆĢ¼·¢ÉśĻĀĮŠ·“Ó¦£ŗ

¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ£ŗK= ”£
¢ŚøĆĪĀ¶ČĻĀ£¬ŌŚ2LŹ¢ÓŠFe2O3·ŪÄ©µÄĆܱÕČŻĘ÷ÖŠĶØČėCOĘųĢ壬10minŗó£¬Éś³ÉĮĖµ„ÖŹĢś11£®2g”£Ōņ10minÄŚCOµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ ”£
£Ø2£©Ä³Š©½šŹōŃõ»ÆĪļ·ŪÄ©ŗĶAl·ŪŌŚĆ¾ĢõµÄŅżČ¼ĻĀæÉŅŌ·¢ÉśĀĮČČ·“Ó¦”£ĻĀĮŠ·“Ó¦ĖŁĀŹ£Øv£©ŗĶĪĀ¶Č£ØT£©µÄ¹ŲĻµŹ¾ŅāĶ¼ÖŠÓėĀĮČČ·“Ó¦×ī½Ó½üµÄŹĒ ”£

£Ø3£©Fe3+ŃĪŗĶAl3+ŃĪŌŚŠŌÖŹÉĻÓŠŗܶąĻąĖʵĵŲ·½£¬ČēÓö¼ī¶¼Éś³ÉÄŃČܵĽŗד³Įµķ£¬æÉÓĆÓŚ¾»Ė®£»Ņ²ÓŠ²»Ķ¬Ö®“¦£¬ČēFe3+¼ČÓŠŃõ»ÆŠŌÓÖÓŠ»¹ŌŠŌ£¬Al3+Ö»ÓŠŃõ»ÆŠŌ”£µ«Fe3+Ö»ÓŠŌŚ¼īŠŌ½éÖŹÖŠ²ÅÄܱ»Ńõ»ÆĪŖFeO2-4£¬ĒėĶź³ÉĻĀĮŠ·½³ĢŹ½£ŗ

£Ø4£©Čō½«ag FeŗĶAlµÄ»ģŗĻĪļČÜÓŚ2mol/LµÄ×ćĮæµÄĮņĖįÖŠ£¬½«ĶłČÜŅŗÖŠ¼ÓČė×ćĮæµÄ6mol/LµÄNaOHČÜŅŗ£¬³ä·Ö·“Ó¦£¬¹żĀĖ£®Ļ“µÓ£¬øÉŌļ×ĘÉÕ£¬³ĘĮæĖłµĆ¹ĢĢåµÄÖŹĮæČŌĪŖag£¬ŌņŌ»ģŗĻĪļÖŠA1µÄÖŹĮæ·ÖŹżĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗø£½ØŹ”ŌĀæ¼Ģā ĢāŠĶ£ŗĢīæÕĢā
 2Fe£Øs£©£«3CO2£Øg£©¦¤H£¾0
2Fe£Øs£©£«3CO2£Øg£©¦¤H£¾0 

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com